1. Background
Snakes are included in the reptilian class, and snakebites are a common cause of mortality among animal bites. Globally, 1.5 to 3 million people are exposed to snakebites each year. More than 100,000 of these snakebites, mostly in the tropics, result in death. Snakebites are usually seen on the hands or feet. The most common victims are agricultural laborers working in rural areas and people taking nature trips (1, 2). Snakebites are a public health concern for countries in the tropics. Therefore, snakebites were included in the list of neglected tropical diseases in 2009 by the World Health Organization (3, 4).
Although Turkey is close to the equator zone, the snake fauna resembles that of continental Europe. Most of these snakes are in the Viperidae family. The most venomous snake species is Vipera lebetina (Blunt-nosed Viper), whereas Vipera ursinii (Meadow Viper) causes most bites in Turkey. Both species are most often seen in the South Anatolian Region, which also encompasses the city of Gaziantep (5).
The most serious snakebites in terms of morbidity are also seen in this region. The arid climate of this region plays a role in settlement of poisonous species (5). There was no clear epidemiological data on our country and region. Our center is one of the important toxicology centers in the region, and 30 - 40 cases of snakebites are treated annually. Mortality due to the widespread use of antivenom is rare. The department of cemeteries did not report deaths from snakebite envenoming for six years (2009 to 2016).
Factors that determine the presentation of toxicity in snakebites consist of the type and amount of the venom, localization of the bite, employed first aid measures, timing of the treatment, developed complications, the presence of comorbidity, and the individual’s immune resistance (6). The clinical presentation is seen as a result of the local and systemic effects caused by the venom (7, 8).
The staging and antivenom treatment is planned according to the staging system that has been developed in accordance with these clinical findings. Grade 3 - 4 extremity bites especially require close follow-up and treatment. Moreover, applying first aid without sufficient knowledge in the case of such bites increases the prevalence of many complications, including the loss of a limb.
2. Objectives
Literature and information concerning snakebites and their treatment are common. However, studies regarding snakebites on fingers and distal extremities and their consequences are very limited. The importance of this study is that it includes only bites on distal extremities (hands, feet, and fingers).
3. Methods
This retrospective study includes patients that presented at the Emergency Department of Gaziantep University Hospital with snakebites on distal extremities (hands, fingers, and feet) between 01/03/2014 and 01/03/2017. The ethics committee approval for the study was obtained from Gaziantep University (Decision no: 2018/01; Date: 13/03/2018). Written consent was obtained from all patients. The data was collected from the archives of the Emergency Department (ED) of Gaziantep University Hospital.
3.1. Inclusion Criteria in Research
• Older than 16 years old;
• Wet snakebites (visible bite, local, and systemic symptoms);
• Distal extremity bites (hands, fingers, feet);
• Visit to ED for control examination.
3.2. Exclusion Criteria in Research
• Dry bites or suspicious bites (visible bite but no envenomation);
• Bites outside of the distal extremities;
• Rejected from participation in the study;
• No visit to ED for control examination.
All patients who met the inclusion criteria were included in this study. The age, gender, date of the event, other diseases in patients' medical history, first aid attempts by patients or relatives, anatomical location of the bite, findings at the time of admission to the ED, administered medical and surgical treatments, complications that developed during follow-up, and the mean duration of hospitalization were recorded for all patients. All patients were requested to return two months after discharge and were re-evaluated for sequelae and complications.
3.3. Treatment Protocol
-Initial assessment: All patients who fulfilled the study criteria were monitored in the ED. After checking ABCD, vital signs were checked. IV cannula was placed, a blood sample was taken, and IV analgesia was given if needed.
- Wound care: Wound care was provided, and tetanus prophylaxis was administered. The wounded extremity was immobilized and elevated. A single dose of a broad-spectrum antibiotic (ceftriaxone 1 g) was given.
- Antivenom treatment: In accordance with the clinical stage of the cases, viper antivenom (Polisera® 10 ml IM/IV; contains immunoglobulin of horse origin against Macroviperalebetina, Montiviperaxanthina, and Viperaammodytes snake venom; produced in Adıyaman, Turkey) was administered.
Antivenom treatment protocol of Emergency Department of Gaziantep University Hospital (9-11)
Grade 0: Visible bite but no symptom; Initial Antivenom dosage (vial): None;
Grade 1 (minimal): Minimal local pain and edema (< 25 cm); Initial Antivenom dosage (vial): None;
Grade 2 (moderate): Moderate pain and edema (25 - 40 cm), nausea, vomiting, and subfebrile body temperature; Initial Antivenom dosage (vial): 2 - 4 vials;
Grade 3 (severe): Severe pain and edema (> 40 cm), petechia, ecchymoses, decreases platelet and fibrinogen, hypotension, tachycardia, and renal and hepatic abnormalities; Initial antivenom dosage (vial): 5 - 9 vials;
Grade 4 (strongly severe): Lethal envenomation, widespread edema, shock, seizures, coma, and renal failure; Initial antivenom dosage (vial): > 10 vials;
After initial antivenom treatment, if systemic symptoms persist (hematologic symptoms > 6 hours, neurological/cardiovascular symptoms < 2 hours), the same dosage of antivenom treatment should be repeated or plasmapheresis should be administered;
- Plasmapheresis treatment: After initial antivenom treatment, plasmapheresis treatment was performed on a patient who had rapidly decreased hematologic parameters and was unstable.
3.4. Statistical Analysis
Changing clinical findings and laboratory parameters during follow-up were recorded. SPSS Windows Ver. 13 software package was used for statistical analysis. Descriptive statistics were provided in numbers and percentages for categorical variables as well as mean (standard deviations) for quantitative variables. The association between categorical variables was analyzed using the Pearson chi-Square Test.
4. Results
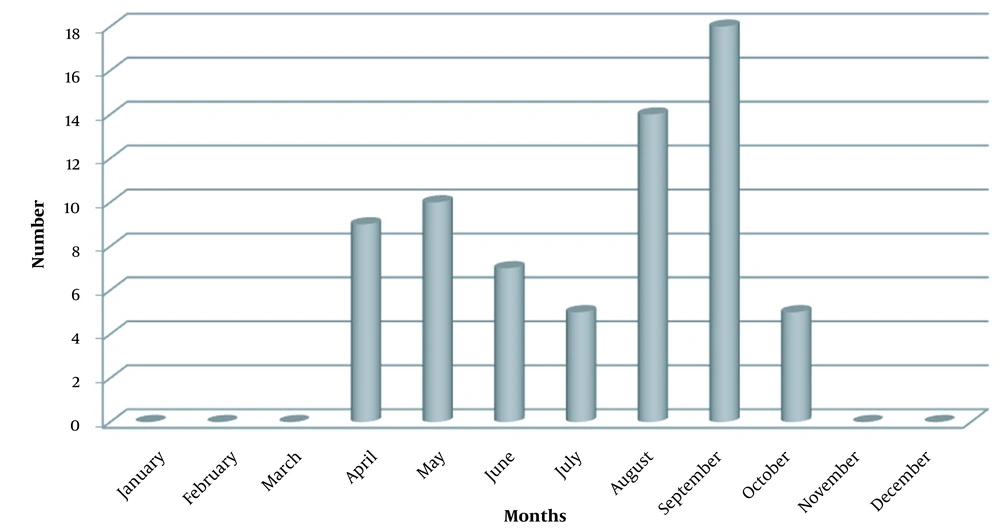
A total of 111 patients presented at the ED with wet snakebites; 68 patients (61.3%) who were bitten on distal extremities were included in this study. Forty-seven patients (69.1%) were male, 21 (30.9%) were female, and the mean age was 43.03 ± 18.13 (range 13 - 79 years). Snakebites most commonly occurred in September (n = 18, 26.5%) (Figure 1). Eleven patients (16.2%) had a chronic disease. Also, 43 (63.2%) patients were grade 1, of whom 67 (98.5%) received only antivenom therapy. One patient (1.4%) had a generalized rash throughout the body immediately after the snakebite. In two patients (2.9%), the bite location was incised by the patient or his/her relatives for first aid purposes. During clinical follow-ups, 2.94 ± 2.5 vials of antivenom (range 0-10) were used per person on average, whereas the mean duration of hospitalization was 2.51 ± 1.5 (range 1 - 8) days (Table 1). Patients were mostly bitten on the right limbs [n = 39 (57.3%)] and the foot of both sides (Table 2).
| Parameter | Values |
|---|---|
| Gender | |
| Male | 47 (69.1) |
| Female | 21 (30.9) |
| Age (y) (min-max) | 43.03 ± 18.13 (13 - 79) |
| Month | |
| April | 9 (13) |
| May | 10 (14.7) |
| June | 7 (10.3) |
| July | 5 (7.4) |
| August | 13 (19.1) |
| September | 18 (26.7) |
| October | 6 (8.8) |
| Chronic disease | |
| Yes | 11 (16.2) |
| No | 57 (83.8) |
| Grade of cases | |
| Grade 0 | 0 (0) |
| Grade 1 | 43 (63.2) |
| Grade 2 | 22 (32.4) |
| Grade 3 | 2 (2.9) |
| Grade 4 | 1 (1.5) |
| Treatment method | |
| Only antivenom | 67 (98.5) |
| Antivenom + plasmapheresis | 1 (1.5) |
| Antivenom dosage (vial) (min-max) | 2.94 ± 2.5 (0 - 10) |
| Hospitalization time (d) (min-max) | 2.51 ± 1.5 (1 - 8) |
a Values are expressed as mean ± SD or No. (%).
| Location | Right (N = 39) (57.3%) | Left (N = 29) (42.7%) |
|---|---|---|
| Thumb | 6 (8.82) | 1 (1.47) |
| Index finger | 10 (14.7) | 6 (8.82) |
| Middle finger | 6 (8.82) | 6 (8.82) |
| Ring finger | 1 (1.47) | 1 (1.47) |
| Little finger | 3 (4.41) | 4 (5.88) |
| Hand | 2 (2.94) | 3 (4.41) |
| Foot | 11 (16.18) | 8 (11.76) |
The most common local symptoms were pain (n = 60, 88.2%) and edema (n = 58, 85.3%). The most common systemic symptom was weakness-dizziness (n = 15, 22%). The most common local symptom was pain (n = 60, 88.2%), followed by edema (n = 58, 85.3%). The most common systemic symptom was weakness-dizziness (n = 15, 22%) (Table 3).
| Finding | No. (%) |
|---|---|
| Local findings (N = 68; 100%) | |
| Pain | 60 (88.2) |
| Edema | 58 (85.3) |
| Movement limitation | 32 (47.1) |
| Ecchymosis | 31 (45.6) |
| Paresthesia | 27 (39.7) |
| Systemic findings (N = 25; 36.7%) | |
| Weakness-dizziness | 15 (22) |
| Hypotension | 11 (16.1) |
| Vomiting-nausea | 10 (14.7) |
| Metallic taste in the mouth | 5 (7.3) |
| Muscular fasciculation | 4 (5.8) |
| Fever | 2 (2.9) |
| Tachycardia | 2 (2.9) |
One patient developed compartment syndrome during ER follow-ups and underwent fasciotomy. The patient developed movement limitations during the follow-up in the second month. In addition, one patient (grade 4 case) received a session of plasmapheresis. The patient who had paresthesia, widespread edema, ecchymosis, and deep hypotension was resistant to antivenom treatment. Records indicated that the patient had movement limitations during the follow-up in the second month.
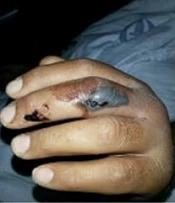
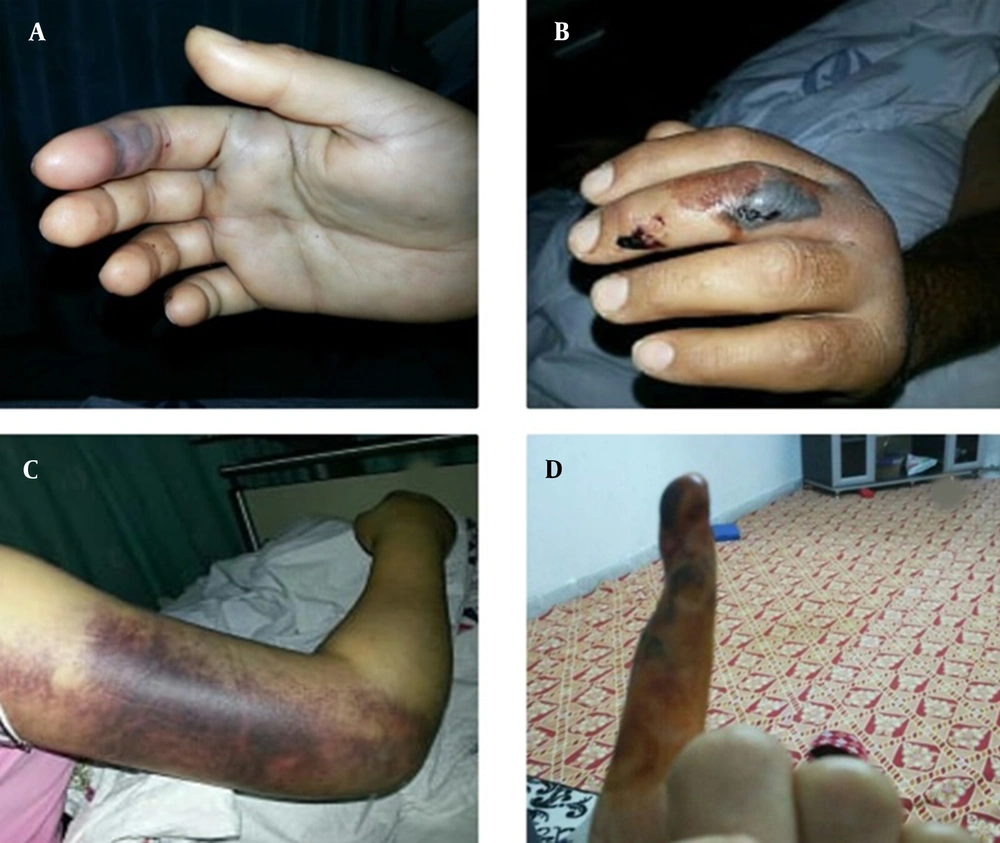
In the physical check-up after two months, 16 (23.6%) patients had complications such as paresthesia, movement limitations, amputation due to necrosis, and trigger finger [paresthesia on the bite localization (10.3%, n = 7), movement limitation on the bite localization (7.4%, n = 5), amputation due to necrosis of the finger (2.9%, n = 2), and trigger finger (1.5%, n = 1)]. Also, one patient was recommended to undergo surgical amputation during follow-ups; however, the patient declined the operation and developed finger atrophy (Figure 2D). There was no statistical association between the anatomical bite location and developed permanent complications (P = 0.906). There was no association between the initial local findings (ie, pain, edema, paresthesia, and movement limitations) and complications (P = 0.959). On the other hand, there was a significant association between the absence of ecchymosis surrounding the bite location upon admission and the absence of sequelae (P = 0.042) (Table 4).
| Sequela 2nd Month | Local Findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Edema | Ecchymosis | Paresthesia | Movement Limitation | ||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Without sequelaen = 52 | 45 (66.15) | 7 (10.3) | 43 (63.3) | 9 (139) | 19 (27.9) | 33 (48.5) | 18 (26.7) | 34 (49.7) | 22 (32.4) | 30 (44.1) |
| Trigger Fingern = 1 | 1 (1.5) | 0 (0) | 1 (1.5) | 0 (0) | 1 (1.5) | 0 (0) | 1 (1.5) | 0 (0) | 1 (1.5) | 0 (0) |
| Paresthesian = 6 | 6 (8.8) | 0 (0) | 5 (7.4) | 1 (1.5) | 5 (7.4) | 1 (1.5) | 1 (1.5) | 5 (7.4) | 2 (2.9) | 4 (5.9) |
| Movement limitationn = 6 | 5 (7.4) | 1 (1.5) | 5 (7.4) | 1 (1.5) | 3 (4.4) | 3 (4.4) | 4 (5.9) | 2 (2.9) | 4 (5.9) | 2 (2.9) |
| Amputationn = 2 | 2 (2.9) | 0 (0) | 2 (2.9) | 0 (0) | 2 (2.9) | 0 (0) | 2 (2.9) | 0 (0) | 2 (2.9) | 0 (0) |
| Atrophyn = 1 | 1 (1.5) | 0 (0) | 1 (1.5) | 0 (0) | 1 (1.5) | 0 (0) | 1 (1.5) | 0 (0) | 1 (1.5) | 0 (0) |
| P-value | 0.806 | 0.958 | 0.042 a | 0.121 | 0.306 | |||||
a Significant at P < 0.05 level, chi-square test.
5. Discussion
The mean age and gender distribution in our study were consistent with other studies conducted in Turkey and other countries, ie, consisted of adult males in the productive age group (8, 12-18). In our study, 69% of patients were male, and the mean age was 43.03 ± 18.13 years. In a study by Elbey et al. (8), 64% of patients were male; in a study by Okur et al. (12), 63% of patients were male; and in a study by Al-Sadoon (15), 81.7% of patients were male. The high number of males can be attributed to the fact that most employees in agricultural enterprises are male.
In our study, bite cases were most commonly seen in September (26%), followed by August. Chang found that most bites (75.7%) occurred between May and November (2). Also, other studies report that bites were commonly seen in August, and the frequency of bites increased in June-October (14-17). It can be explained by the fact that snakes are cold-blooded animals and are more active in hot months, and agricultural activities are more intense in these months.
Two patients were bitten by a snake twice. One of them was bitten twice on the middle finger of the right hand, whereas the other was bitten twice on the lateral side of the left foot. In a study on 87 patients, Valenta et al. reported that one patient was bitten twice on the lower and upper extremities (13). Another study showed that a patient, who presented with neurological and respiratory signs in Guinea, was bitten by a snake twice (19).
Ultimately, these patients fully recovered and were discharged after antivenom treatment. However, one of these patients required surgical amputation due to developing necrosis localized near the bite mark on the middle finger. We assume that this situation is related to the fact that the snake bit the patient twice and released all of its venom due to the provocation.
Allergic and anaphylactic reactions due to antivenom administration have been reported in the literature, whereas allergic reactions due to snakebites were less common (20-23). In a study by Shahmy, 31% of patients who received antivenom developed anaphylactic reactions (21). Hypersensitivity is not a common symptom of snake bites, but it can occur with recurrent exposure. Following a case report design, de Medeiros et al. described a patient who worked with poisonous snakes for 13 years and had a hypersensitivity reaction after a snakebite (23). In this study, one patient had generalized urticarial lesions after the snakebite, and the patient did not develop any allergic or anaphylactic reactions after antivenom administration.
Studies conducted earlier revealed that incorrect first-aid practices, such as applying a tight tourniquet, sucking, cutting, and bleeding, were applied in addition to herbal methods with unknown contents before admission to the hospital. Studies conducted in Turkey determined that 56.7% and 92.1% of the cases used a tight tourniquet, sucking, cutting, and bleeding in the prehospital setting. Amputation and cellulitis have been reported as common complications of these inappropriate practices. Another study conducted in India reported the death of a patient who received a first-aid tourniquet (2, 17, 24).
Michael et al., who also included emergency physicians, found that 75.7% of the physicians had knowledge of first-aid applications for snakebites (25). Our study also revealed improper first-aid applications, that is, the bite location was incised in two patients. Unlike other studies, none of the patients used tourniquets.
Elbey et al. found that lower extremity bites were more common in total, whereas individually, the bites were most commonly seen on the right hand (8). Karakus et al. and Altun et al. found that the prevalence of bites on the upper extremity was higher (52.8% and 52%, respectively) (26, 27). Valenta et al. found that all bites were localized on the upper extremity (13). A North American study by Ruha observed a lower rate of extremity bites, which was 54% of all bites, wherein 27% of the patients who had lower extremity bites were not wearing shoes (28). Chang et al. observed most of the bites (44%) on fingers; 38.2% of these bites consisted of toe bites, whereas 5.8% of these bites were seen on the right index finger (2). In another study conducted in West Bengal, lower extremity bites, the majority of which consisted of bites on the right foot, were more commonly encountered (14). Al-Sadoon observed that lower extremity bites were again more common (15-17). Bites on the lower extremities may result from the fact that agricultural laborers, especially in the Far East, do not wear shoes. Considering that upper extremity snake bites were reported more frequently in the studies conducted in Turkey, it may be asserted that bites on hands result from not wearing protective gloves while working in the field. In our study, bites were most commonly seen on the index finger of the right hand. A similar result can be seen in the distribution of bites on hands and fingers in a study by Al-Sadoon (15).
Local reactions to snakebites were reported most commonly as pain, edema, and ecchymosis, which is consistent with our study (2, 15, 17, 18, 26, 27, 29). Ozay et al. underlined that the presence of ecchymosis was a risk factor for the development of complications (29). In our study, initially, there was a statistical association between the presence of ecchymosis in the periphery of the bite and the development of complications.
The frequency of systemic symptoms (ie, nausea, vomiting, syncope, and hypotension) is reported, and the most common symptoms were weakness and fatigue (2, 8, 13, 17, 18, 26, 27). Sarkhel et al. pointed out that severe envenomation was characterized by hypotension, shock, and anaphylactoid reaction (30). According to a study by Chang et al., there was a significant association between the presence of systemic signs and the duration of hospital stay (2). In our study, we found that weakness-dizziness (22%), hypotension (16.1%), and vomiting-nausea (14.7%) were the most common systemic symptoms.
The researchers applied plasmapheresis to a patient whose edema and ecchymosis were rapidly spreading despite the clinical follow-up and treatment in the ER. In a study by Valenta et al., plasmapheresis was applied to a patient who developed acute kidney injury, and the results favored the patient (13). In our study, one patient received a session of plasmapheresis. The patient had paresthesia, widespread edema, ecchymosis, and deep hypotension.
Previous studies also mentioned patients who developed compartment syndrome in clinical follow-ups and underwent fasciotomy (2, 8, 13). Chang et al. observed four patients who were bitten on the right foot, leg, and digits and underwent fasciotomy due to developing compartment syndrome (2). Elbey reported that 1.9% of the patients developed compartment syndrome, whereas another study mentioned a patient who developed compartment syndrome on the upper extremity underwent fasciotomy followed by mannitol treatment (8, 13). Surgical amputation was applied to two patients in a study of 25 patients by Altun et al., whereas Elbey et al. observed that amputation was applied to 2.3% of the patients, which is consistent with the results of our study (8, 27). In our study, one patient underwent fasciotomy of the upper extremity, and amputation was performed on two patients due to necrosis of the finger.
In the literature, the mean amount of antivenom administered to the patients, duration of hospital stays, and complications exhibited regional differences. the mean amount of antivenom administered to the patients, duration of hospital stays, and complications exhibited is higher in Latin America, Far East Asia, and Africa, but it is lower in Turkey and Europe. Researchers surmise that this issue roots in the fact that snake fauna primarily consists of snake species with low venom toxicity. Various mortality rates were reported in other countries (2, 8, 15-17, 26, 28, 31). However, Chang reported no mortality (2). Elbey also reported no mortality and revealed that the most common complication was amputation (4.67%) (8). In our study, the most common complication in control examinations after 2 months was paresthesia on the bite localization. However, this can be interpreted as a subjective complaint. In this study, there was no association between the complications seen on hands and feet and the anatomical localization after snakebite. Snakebites did not cause mortality in any of the patients.
It is necessary to mention some limitations and biases of our study, including following a retrospective design and a small sample size. Also, the study population was limited since body parts—except for hands and feet—and dry bites were excluded. The duration between the snake bite and admission to the emergency department, which affects the clinical situation, could not be calculated.
5.1. Conclusions
Snakebites are an occupational health and safety problem for agricultural laborers, in addition to being a public health concern for developing countries. In this study, 61.3% of patients presented with bites on distal extremities like fingers, hands, and feet. Hence, this study focused on hands, fingers, and feet because these parts can be protected (ie, preventing bites) by wearing shoes and protective gloves. Also, there was no mortality due to extremity bites. The most common long-term complications were paresthesia and movement limitations. It is recommended that high-risk populations (such as agricultural laborers, nature travelers, and documentary teams) be taught appropriate first-aid practices after snake bites.