1. Background
Among the significant characteristics of aging, some changes like estrogen deficiency and an increase in its absorption are the factors that demonstrate the reduction of bone density in women (1). The annual bone density reduction in menopausal women is estimated at 0.6% in 60 to 69 years old women, 1.1% in 70 to 79 years older women, and 2.1% in women higher than 80 (2). Numerous factors such as genetics and environment affect bone density. Genetic factors cannot be changed, but correcting some variables like lifestyle, physical activities, and suitable diets effectively stimulates increased bone density (3). Among the studied elements practical on the metabolism and skeletal strength, consuming calcium and vitamin D supplements is suggested as a strategic procedure to avoid premature bone loss in menopausal women (4). Some studies have shown that calcium existing in the body cannot be consumed. Therefore, applying procedures like doing exercise will be a suggested method to facilitate calcium consumption (5). It seems to do exercise influences skeletal structure in three different procedures. The first is the direct effect imposed by transferring biological signals of mechanical receptors on bones. Mechanical signals are transmitted through the osteocytic network to the epithelial cells of the bone. They secrete paracrine agents such as prostaglandins and insulin-like growth factor, which results in the proliferation of osteoblast cells, resulting in the synthesis of bone matrix (6). The second is the direct effect of muscle mass, which influences mechanical receptors peripherally, and it is considered the last mechanism of indirect hormone effects (7). The third one studies the effects of hormones, of which parathyroid hormone (PTH) is the most important. It is one of the hormones that play an influential role in stimulating, forming, and absorbing bone. Moreover, PTH is a central regulator of bone metabolism and regulates the concentration of extracellular fluid calcium in the physiological range (8). The secretion of PTH is regulated by the calcium level in the blood, in the form of negative feedback, and exercise indirectly affects the secretion of PTH by affecting the calcium level in the blood and secreting some myokines (9).
Alkaline phosphatase (ALP) is one of the most critical indicators of bone formation, and changes in its serum level will reflect skeletal changes (10). ALP indicates the activity of osteoblast cells. Osteoblasts are an essential source of ALP, so increasing the level of ALP is associated with the ability of osteoblasts to form bone and reducing the level of ALP by destroying or reducing bone formation (11). The effect of exercise and physical activity on bone metabolism has been shown in many studies by examining PTH and ALP levels changes (9, 11, 12). In contrast, the effects of physical activities and the efficiency threshold of these activities on hormones have not been sufficiently studied. As Maimoun et al. evaluated the PTH density in young cyclist men, there was a stimulation threshold for the duration and intensity of exercise on PTH activity (13). The efficiency threshold of physical activities on ALP increased in four days after doing exercise (14). Although exercise and physical activities are widely suggested as efficient and powerful non-medical procedures to avoid osteoporosis, their effects on bones are still controversial. While some evidence claims the increase of bone density induced by physical activities in young men, its efficiency in middle-aged and older people is questionable. In addition, it is unclear what physical activity is the most suitable to fulfill the intended results and how long should be spent doing activities. SIOMMMS (Societa Italiana del Osteoporosis Metabolismo Minerale delle Malattie dello Scheletro) has recently reviewed its former suggestions and advised 30 min daily physical activities like walking. At the same time, ACSM (American College of Sports Medicine) suggests doing more strenuous physical activities like running, jumping, and walking up and down the stairs three to five times a week for 30 to 60 minutes (15). In general, even though physical activities are profitable in improving bone density, much more attention must be paid to three significant factors of type, severity, and duration during the prescription (16).
2. Objectives
We aimed to study the response threshold of ALP and PTH to resistance exercise and calcium and vitamin D supplements in menopausal women.
3. Methods
This study is a quasi-experimental one with pre-test and post-test forms along with a control group. Following the related procedures to approving the research project and its verification in the ethics committee of the University of Sistan and Baluchestan with IR.USB.REC.1398.024 ID, the authors started administrative procedures. Using G-Power software version 3.1.9.2, the sample size for the present study (two groups with five measurement steps), with an effect size of 0.8 and α error of 0.05, test power of 0.95, 16 people (8/group) was considered. At first, a declaration was distributed in gyms located in Zahedan, and menopausal women were invited to participate in the study. After identifying the participants, those with the defined inclusion criteria such as having no former history of cardiovascular diseases, diabetes, asthma, having no former break in bones, no smoking history, and those with the age range of 50 to 60 years were included in the study. The exclusion criteria were overlooking researcher guidelines, regular exercise programs, and regular consumption of supplements. It is noteworthy to mention that ethical and moral regulations of the study like informed satisfaction, following privacy and secrecy, supporting the participants against pressures, possible dangers, and physical tensions, and making them aware of the results were completely followed. In addition, in order to provide a relative control over the nutrition, a listed diet was prepared (17). The participants were suggested to avoid any other supplements containing calcium. Blood sampling was repeated in five steps in control and experiment groups, so the first samples were taken two hours before the first exercise session and the second samples 24 hours after the last exercise session in the first week. The third samples were taken 24 hours after the last exercise session during the second week. The fourth samples were taken 24 hours after the last exercise session of the third week. Finally, the fifth samples were gathered 24 hours after the last exercise session of the fourth week. It should be mentioned that all blood samplings were done after 10 to 12 hours of night fasting in the same condition. The participants were asked to be present in the Imam Ali hospital laboratory 30 minutes before starting the sampling procedure. After 15 min rest, five cc blood samples were taken from their brachial vein. The samples were immediately centrifuged to separate serum. Then the serum was stored at -80ºC until the serum levels of PTH were measured by ELISA method and ALP by photometric method.
3.1. Exercise Protocol
About a week before starting the exercise protocol, the participants were asked to visit San Sovan gym located on Khayyam Street to get familiar with sports equipment and intended movements during their exercise. Their regular exercise is determined as one repetition maximum in barbell bench press, instep, lying leg press, curl, standing barbell, and length stretch. The experimental group started its exercise session three times a week for four weeks, including 10 min warm-up, 45 min for the primary procedure, and five min for cooling down. The exercise schedule is credited and studied one planned based on the instructions of the U. S. diabetes committee and medical science American college. In these guidelines, it was suggested to prescribe three sets of 8 - 10 min repeated exercise three times a week in all major muscle groups. Therefore, resistance movements (barbell bench press, instep, lying leg press, curl, standing barbell, and length stretch) were performed in three sets with six repetitions, and an intensity of 55% 1RM was performed the first week to 65% 1RM in the fourth week. Participants rested between the sets and the exercise for two and five minutes, respectively (18).
3.2. Supplementation
According to the USPTF (the United States preventive task force) declaration in 2013, no evidence was found to show that less than 1,000 mg calcium and less than 400 IU vitamin D in a day are beneficial (19). Therefore, participants in the experiment group were prescribed 1,500 mg calcium and 600 IU vitamin D (20). They consumed them during the study period daily. Due to the lack of cooperation of the subjects in recording the food consumed during the research period, we asked the participants to avoid using any other supplements, including calcium and vitamin D, and to change their daily diets and their consumed supplement dose.
3.3. Statistical Analysis
First, the normal distribution of the data was tested and confirmed using the Kolmogorov-Smirnov (K-S) test. Then, using SPSS version 19 to analyze the collected data, an independent sample t-test and a repeated-measures analysis of variance (ANOVA) were performed along with an LSD post hoc test. Data were presented as mean ± SD, and the significance level was set at P < 0.05. One researcher performed all stages of this study, and the same researcher performed all measurements and data collection.
4. Results
Table 1 represents the personal information of participants. In Table 2, ALP and PTH intake are presented in five steps of measurements.
| Variables | Control Group | Experimental Group | P-Value a |
|---|---|---|---|
| Height (cm) | 162 ± 3.28 | 158 ± 4.34 | 0.164 |
| Weight (kg) | 69.75 ± 7.90 | 65.75 ± 5.49 | 0.262 |
| BMI (kg/m2) | 26.30 ± 2.60 | 26.21 ± 2.45 | 0.478 |
| Age (y) | 54.12 ± 2.16 | 52.50 ± 1.30 | 0.096 |
Participants’ Height, Weight, Age, and Body Mass Index
| PTH Pm/L | ALP IU/L | |||||
|---|---|---|---|---|---|---|
| Control Group | Experimental Group | P-Value b | Control Group | Experimental Group | P-Value b | |
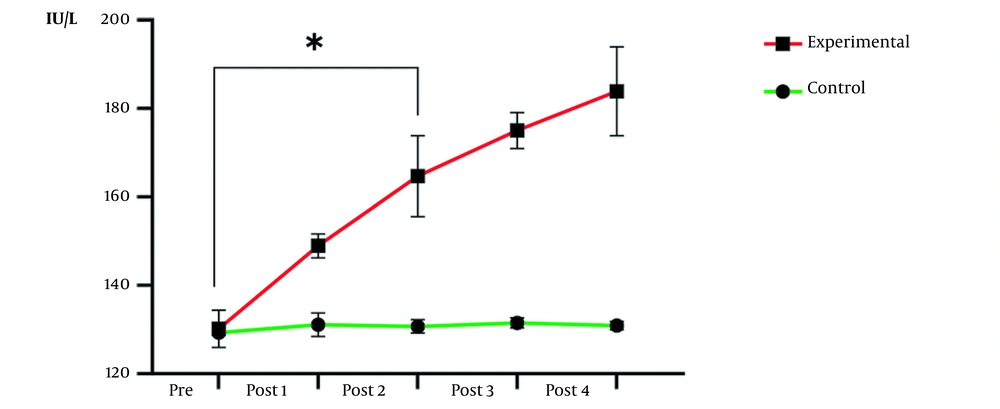
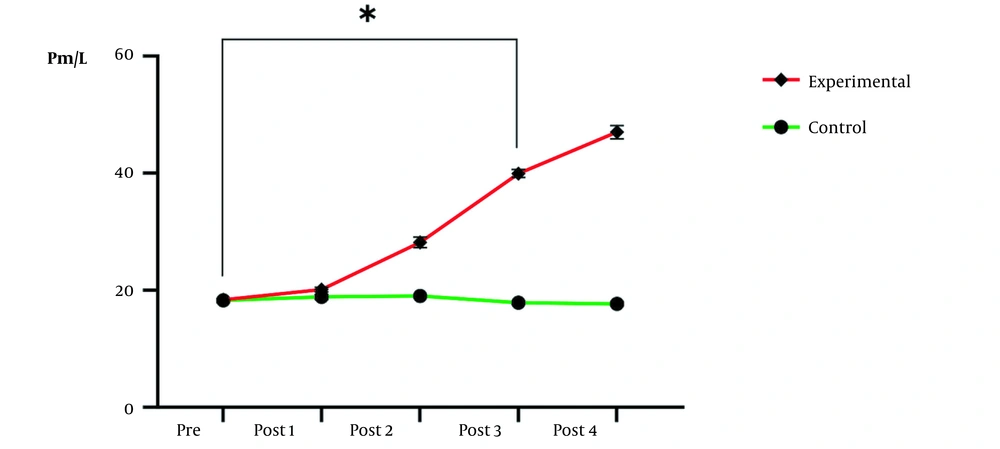
| Pretest | 18.25 ± 0.70 | 18.37 ± 0.63 | 0.560 | 129.30 ± 1.53 | 130.18 ± 2.12 | 0.578 |
| Post 1 | 18.85 ± 1.22 | 20.12 ± 2.89 | 0.982 | 131.12 ± 1.83 | 148.94 ± 7.10 | 0.096 |
| Post 2 | 19.00 ± 1.06 | 28.91 ± 4.56 | 0.063 | 130.73 ± 3.94 | 164.68 ± 6.89 | < 0.001 c |
| Post 3 | 17.87 ± 2.29 | 39.94 ± 3.09 | < 0.001 c | 131.50 ± 4.41 | 175.02 ± 5.78 | < 0.001 c |
| Post 4 | 17.70 ± 1.20 | 47.00 ± 1.02 | < 0.001 c | 130.89 ± 3.01 | 183.85 ± 4.03 | < 0.001 c |
Alkaline Phosphatase and Parathyroid Hormone Levels a
Effect of resistance training and taking vitamin D and calcium supplements on serum ALP and PTH levels:
The results of repeated-measures ANOVA indicated the effect of time (P = 0.02) and group (P < 0.0001), as well as the interactive effect of time and group (P < 0.0001) on ALP level, were significant. In other words, the increase in ALP in the experimental group compared to the control group in the five stages of measurement was statistically significant (Figure 1). Due to the importance of average differences in measurements, post hoc test results clarified the second week was the threshold of significant changes (P = 0.001).
Also, the results of repeated measures ANOVA indicated that the effect of time (P = 0.004) and group (P < 0.0001), as well as the interactive effect of time and group (P < 0.0001) on PTH level, were significant. In other words, the increase in PTH in the experimental group was statistically significant compared to the control group in five stages of measurement (Figure 2). Due to the importance of average differences in measurements, post hoc test results clarified that the third week was the threshold of significant changes (P < 0.0001).
5. Discussion
The results have proved the effects of resistance exercise and consumption of calcium and vitamin D supplements on ALP and PTH levels in menopausal women. Obtained findings of a post hoc test on ALP index show its increase following the first-week resistance exercise and vitamin D and calcium consumption; however, it was not significant. Its significant increase occurred after two weeks of resistance exercise along with calcium and vitamin D supplementation. The PTH index obtained results of a post hoc study proved a significant augment of its level following three weeks of resistance exercise and supplement consumption. These results were inconsistent with those obtained by Tartibian and Moutab, who announced obvious augment of PTH and ALP levels following six weeks of strenuous aerobics exercise (12). The studied participants in their study consisted of a group of menopausal women who had received calcium and vitamin D supplements accompanied by doing strenuous exercise. It is generally shown that bone metabolic response to the duration and severity of exercise has a stimulation threshold (13). Thus, because the participants were younger, if the exercise protocol intensity was higher in the study by Tartibian and Moutab (12). The time to increase PTH and ALP levels was probably reduced. Previous studies on PTH have shown that its density increases during strenuous physical activities (at least 15 percent higher than the stimulation threshold) without any change in blood calcium level (8). In this study, the severity of muscle contraction was high. In addition, Gomarasca et al.’s study on the skeleton and skeletal muscle expressed that skeletal muscle can affect skeletal metabolism in a non-mechanical way (in a hormonal way) (21). Therefore, to prove the increase of PTH level while using calcium, it will be claimed that it would be claimed that the increase does not make it blood calcium levels. Other factors like myokines possibly make it. In addition, other factors like catecholamines and acidosis levels are involved in PTH secretion (22), which will be another justified reason for its augmentation.
On the one hand, it has been shown that ALP changes can demonstrate the bone formation process. Sahrir, in their review study, have shown that 32 weeks of aerobic exercise with a minimum 30-joule workload in a week was necessary to increase skeletal mass in rats (6). In order to better understand the results, it will be announced that two factors -extreme severity of exercise and calcium consumption- have induced the progressive augmentation of this process, as it has been shown that calcium consumption increases the speed of ALP activity (22). On the other hand, we measured serum ALP levels. It is noteworthy that half of the existing ALP in the blood is related to bones, and another half is related to the liver (23). It is worth mentioning that increasing bone density is a step happening after increasing ALP serum levels. Also, the results of another study on human beings have increased ALP serum levels following six weeks of circuit training with dumbbells and rubber bands in 19 to 25 years old men (24). They studied young men while our research was on older women. This difference between the two groups of participants will be justified considering their age average, as a bone reaction to exercise varies in different age ranges (6). Another study showed that bone resorption reduction was observed in women with osteoporosis who walked mildly for at least three months (25). Differences in results are probably due to the difference in the severity of activities and disease levels. Both were stopping the disease process and starting the improvement of physical condition are time-consuming. According to the obtained results, doing resistance exercise and consuming calcium and vitamin D supplements positively affected serum ALP levels. Alkaline phosphatase levels increased after two weeks and induced transforming mechanical pressure into biochemical signals and forming or mineralizing bones (mechanical pressure transformation hypothesis) (26). For PTH, it will be declared that to start its activity and its effects on bone metabolism, it is necessary to do three weeks of resistance exercise and consume calcium and vitamin D supplements.
Based on our data, this study is the first one exploring ALP and PTH effects after one-week involvement. At the same time, our findings are solely limited to inactive menopause older women studied for four weeks. More studies would be done on different participants based on different protocols without supplements and for a larger group of statistical population. One of the significant limitations of the present study was that we did not measure the baseline levels of the research variables, so we could use statistical methods to show how the levels of the variables changed during the research. However, changes in the levels of variables were considered in each stage of the intervention compared to other stages of the study, and this evaluation performed well.
5.1. Conclusions
Based on the obtained results, it may be declared that the response threshold of ALP to resistance exercise and daily consumption of calcium and vitamin D in menopausal women is the second week, while it is the third week for PTH.