1. Background
Systemic lupus erythematosus (SLE) is a polymorphic autoimmune disease that primarily affects young women (1, 2). Its highest incidence is reported in individuals aged 15 - 40 years (3). Lupus is rare and more insidious in the elderly than in younger patients. It is defined by its onset after the age of 50 years. The incidence of this disease is estimated to be 4 - 16% in most studies (1, 4-11).
Several studies have examined late-onset SLE, suggesting that the age of onset alters the clinical expression of the disease in terms of onset, clinical presentation, organ involvement pattern, and serological findings. In this regard, different modes of presentation, including arthritis and lung disease, have been described. The most consistent features in elderly patients have been the higher frequency of arthritis and the lower frequency of Raynaud’s disease, neuropsychiatric involvement, and severe kidney injury. Serologic abnormalities have also been reported to be different in elderly patients.
2. Objectives
The present study aimed to define the clinical, biological, and prognostic specificities of 12 cases of SLE emerged after the age of 50 years and compare these data with early-onset SLE cases ad those described in the literature.
3. Methods
This retrospective multicenter study included consulting patients’ files. We identified 12 patients with SLE at the age of 50 years and above (group A) from 2006 to 2019 in two Western Algeria hospitals: the University Hospital of Oran (EHUO) and the University Hospital Abdelkader Hassani of Sidi Bel Abbes (CHU-SBA). We also included 191 patients with onset SLE at the age of 13 - 49 years (group B). In total, two hundred and three patients were included in our study. Group A encompassed postmenopausal women. Inclusion criteria were the presence of at least four criteria from the 1982 American college of rheumatology (ACR) SLE classification, as revised in 1997 (12, 13). The criteria approved by SLE and SLEDAI (lupus disease activity index) were used (14).
Late-onset SLE is defined by the age of first symptoms retrospectively attributable to SLE. The clinical-biological characteristics (at the initial phase and during the course) and immunological, therapeutic, and comorbidities features have also been described in this regard.
3.1. Statistics
We used the Statistical Package for the Social Sciences (SPSS 22.0 Inc, Chicago, IL, USA) for all conventional analyses. Means and standard deviations were calculated for the age of onset, age at diagnosis, duration between first symptoms and the disease, and follow-up duration. The Person chi-squared test with Fisher’s correction was used to compare the numbers. In this study, the significance level was set as P ≤ 0.05.
4. Results
We studied the clinical records of 203 patients with SLE (90.6% female and 9.4% male), with a F/M gender ratio of 9.86/1. The mean age of the disease onset was 29.47 ± 11.24 years. The mean duration of follow-up was 12.7 ± 9.18 years.
In 12 patients, the age of disease onset was 50 years and above (≥ 50 years). This group of patients (A) was compared with 191 patients in group B (disease onset, 13 to 49 years). The mean age of the disease onset in group A was 59.17 ± 11.10 years (range: 50 and 89 years), and the mean age of clinical diagnosis was 67.67 ± 10.95 years. Ten patients (83.3%) were postmenopausal women, and two participants were men (16.7%). The gender ratio of female to male was 5 to 1 and 10 to 1 in groups A and B, respectively. The mean duration of follow-up was 8.5 ± 4.33 years in group A and 13.02 ± 9.34 in group B. (Table 1).
| Group A (≥ 50 y, n = 12) | Group B (< 50 y, n = 191) | P-Value | |
|---|---|---|---|
| Gender | 0.312 | ||
| Male | 2 (16.7) | 17 (8.9) | |
| Female | 10 (83.3) | 174 (91.1) | |
| Age at onset (y) | 59.17 ± 11.10 | 27.61 ± 8.25 | < 0.001 |
| Age at diagnosis (y) | 67.67 ± 10.95 | 40.75 ± 12.45 | 0.044 |
| Duration from onset to diagnosis (y) | 8.5 ± 4.33 | 13.02 ± 9.34 | 0.868 |
Demographics Data of SLE Patients by the Age of Onset a
Table 2 presents the frequency of initial manifestations for the two age groups. The most frequent clinical manifestations of group A were arthritis (91.7%), malar rash (58.3%), photosensitivity (41.7%), Hematological involvement (58.3%), and lupus pneumonia (33.3%). Lupus nephritis was found in a single patient (8.3%) in group A and 50 patients (26.2%) in group B. Moreover, the mortality rate was significantly higher in the younger group than in the older group (P = 0.024).
| Group A (≥ 50 y, n = 12) | Group B (< 50 y, n = 191) | P Value | |
|---|---|---|---|
| General signs | |||
| Fever | 1 (8.3) | 20 (10.5) | 0.793 |
| Asthenia | 3 (25) | 80 (41.9) | 0.366 |
| Weight loss | 1 (8.3) | 37 (19.4) | 0.471 |
| Anorexia | 1 (8.3) | 18 (9.4) | 1.000 |
| Dermatological disorders | 7 (58.3) | 138 (72.3) | 0.329 |
| Malar rash | 5 (41.7) | 106 (55.5) | 0.384 |
| Photosensitivity | 5 (41.7) | 78 (40.8) | 1.000 |
| Oral ulcer | 4 (33.3) | 30 (15.7) | 0.121 |
| Alopecia | 2 (16.7) | 39 (20.4) | 1.000 |
| Arthritis | 11 (91.7) | 143 (74.9) | 0.300 |
| Pericarditis | 1 (8.3) | 12 (6.3) | 0.932 |
| Renal involvement | 1 (8.3) | 50 (26.2) | 0.302 |
| Lupus pneumonitis | 4 (33.3) | 64 (33.5) | 0.820 |
| Neuropsychiatric | 0 | 25 (13.1) | 0.317 |
| Haematological disorder | 7 (58.3) | 139 (72.8) | 0.351 |
| Raynaud’d syndrome | 1 (8.3) | 51 (26.7) | 0.318 |
| Sjogren’s syndrome | 2 (16.7) | 8 (4.2) | 0.142 |
| APLS | 1 (8.3) | 20 (10.5) | 0.880 |
| Gastrointestinal damage | 0 | 5 (2.6) | 0.694 |
| Mortality | 6 (50) | 5 (2.6) | < 0.001 |
Clinical Data of SLE Patients by the Age of Onset a
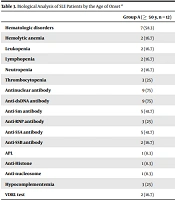
Table 3 lists the serological results as a function of age. Using the SLEDAI criteria, we compared the severity of SLE between the two groups. In this table, the ACR criteria numbers are also included. In group A, the serological analysis showed the positivity of anti-dsDNA antibodies at 75 and 41.7% for each of anti-Sm anti-SSA, and 25% hypocomplementemia. No significant difference was noticed regarding the incidence of autoantibodies (dsDNA, Ro, La, Sm, and RNP).
| Group A (≥ 50 y, n = 12) | Group B (< 50 y, n = 191) | P Value b | |
|---|---|---|---|
| Hematologic disorders | 7 (58.3) | 139 (72.8) | 0.351 |
| Hemolytic anemia | 2 (16.7) | 17 (8.9) | 0.312 |
| Leukopenia | 2 (16.7) | 42 (22) | 1.000 |
| Lymphopenia | 2 (16.7) | 63 (33) | 0.345 |
| Neutropenia | 2 (16.7) | 8 (4.2) | 0.111 |
| Thrombocytopenia | 3 (25) | 44 (23) | 1.000 |
| Antinuclear antibody | 9 (75) | 170 (89) | 0.156 |
| Anti-dsDNA antibody | 9 (75) | 117 (61.3) | 0.541 |
| Anti-Sm antibody | 5 (41.7) | 58 (30.4) | 0.521 |
| Anti-RNP antibody | 3 (25) | 38 (19.9) | 0.711 |
| Anti-SSA antibody | 5 (41.7) | 67 (35.1) | 0.758 |
| Anti-SSB antibody | 2 (16.7) | 30 (15.7) | 1.000 |
| APL | 1 (8.3) | 24 (12.6) | 1.000 |
| Anti-Histone | 1 (8.3) | 15 (7.9) | 1.000 |
| Anti-nucleosome | 1 (8.3) | 10 (5.2) | 0.497 |
| Hypocomplementemia | 3 (25) | 65 (34) | 0.754 |
| VDRL test | 2 (16.7) | 7 (3.7) | 0.092 |
Biological Analysis of SLE Patients by the Age of Onset a
Early-onset patients (group B) showed a significantly higher prevalence of neutropenia (P = 0.053) than late-onset patients (group A).
The presence of other pathologies associated with lupus disease has been noticed in groups A, and B as 41.7% vs. 15.2% of the participants had arterial hypertension (hypertension), 16.7% vs. 4.2% of the cases suffered from Gougerot Sjogren syndrome (SGS), and 16.7% vs. 6.8% of the participants had rheumatoid arthritis (RA). Diabetes was observed in only one case (8.3%) and in 12.6% of the patients in group B. Other autoimmune pathologies, such as dermatopolymyositis (DPM), psoriasis, Hashimoto’s thyroiditis, were observed in the two groups (75% vs. 32.5%). A significant correlation between the presence of arterial hypertension was found between the two groups (41.7%. vs. 15.2%, P = 0.056).
Table 4 indicates that cerebrovascular accident (stroke) as the leading cause of death in both groups. A significant difference was found in the incidence of CVA, renal failure, and septic shock with P = 0.003, P = 0.018, and P = 0.059, respectively.
5. Discussion
Late-onset SLE represents a specific disease subgroup, as most cases are noted in postmenopausal women (15). It begins at the age of 50 - 65 years old and above (4, 16, 17). Late SLE is rare as it affects only 12 - 18% of the population (4, 18, 19).
Out of 203 SLE patients, twelve patients (9.4%) of developed the disease after the age of 50 years. This frequency is similar to that reported in some other studies (7 - 18%) (4, 7, 20, 21). A female predominance in the group of the elderly was noted in our cases as well as other cases reported in the literature (19, 21, 22). However, some reports have suggested that the female predominance is not so marked in the elderly (7, 20, 23). In general, other studies have reported a significantly higher incidence of male lupus in this age group (1, 7, 24-26). The gender ratio of women to men declined in the elderly group, as revealed in previous reports. In our study, the F/M ratio in group A was similar to the ratio reported in other studies (19, 27). However, it was lower than that of the younger group, 10.23 : 1 (group B).
Regarding the length of time from onset to diagnosis, the younger group has a significantly longer duration than the older group. (8.5 vs. 13.02 years). On the other hand, many previous reports demonstrated that this duration was longer in the older than in, the younger patients (19, 28, 29).
Numerous studies have suggested that patients with late-onset lupus differ from those with early-onset lupus in their clinical presentation, organ involvement pattern, and disease severity. Accordingly, different conclusions were drawn, possibly due to racial differences (4, 17).
The clinical course of late-onset SLE is considered milder. In patients with late SLE compared to patients with SLE at an earlier age, skin manifestations, nephritis, neuropsychiatric, and cardiac manifestations were less frequent (4, 19, 21, 30). In our study and many other studies, malaria erythema was also less common in older individuals with SLE (1, 6-9, 29, 31, 32). In contrast, photosensitivity is more frequent in the present study and Dimant et al.’s (5) study. Likewise, oral ulcerations are more frequent in this study and the study by Chen et al. (28). These differences can be justified by differences in sun exposure in different countries.
Regarding the late SLE cases, we observed a higher incidence of lung involvement and Sjögren’s syndrome, similar to other studies (4, 19, 21, 30, 33). Likewise, arthritis was more common in the elderly than in younger patients. This finding was in contrast with some other studies (4, 19, 21, 30, 34, 35).
Similar to the present study, Madisson (19) reported the higher prevalence of cardiovascular complications in a group of 86 patients with late-onset SLE (8.3% vs. 6.3%). Regarding clinical characteristics, no significant difference was found between the two groups. as the same was also noticed for biological analyses, except neutropenia (P = 0.053) (Table 3). In contrast, Wilson et al. (30), Ballou et al. (20), and Chen et al. (28) found a significant difference between patients with late SLE and younger participants in terms of three immunological criteria (anti-dsDNA antibody, hypocomplementemia, and anti-RNP antibody). Likewise, hemolytic anemia has been more common in the elderly, as reported in three other recent studies (11, 31, 36). A higher positive level of anti-SSA and anti-SSB antibodies in the case of late SLE was noted in the present cases and several cases in the literature (21, 25, 34, 36, 37). Anti-Sm antibodies and anti-RNP were also found at a high level in the late SLE cases in the present study. In contrast, these antibodies are found at low frequency in other studies (1, 19, 25, 38). Our patients with late-onset lupus also have less frequent hypocomplementemia; however, this is not constant compared to younger patients (5, 16, 19, 20, 30, 34, 39), which is not surprising given the less severe manifestations of the disease. The same findings are also revealed in several other studies.
Concerning the disease severity, no significant difference was noticed between groups A and B. The patients with late SLE showed a high mortality rate (P = 0.024). This finding is consistent with those reported by Chen et al. (P = 0.022) (28) and some other researchers (8, 29, 38).
In both groups, stroke was the common cause of death and was mainly associated with the presence of high blood pressure (hypertension). Similarly, Bertoli et al. (38) reported cardiovascular pathologies as the leading cause of death in patients with late-onset SLE. In contrast, in their studies, Chen et al. (28) and Pu et al. (29) reported septic shock as the leading cause of death.
The small sample size of our late SLE patients was a limiting factor in the present study. Accordingly, large-scale studies are recommended to further examine the molecular physiopathology of this disease.
5.1. Conclusions
The low prevalence of late SLE and the presence of comorbidity with similar symptoms in the elderly patients make the diagnosis difficult. Accordingly, further attention to this patients group is needed to avoid diagnostic delays.