1. Background
Thalassemia is a hereditary blood disorder that over 300 million people of South Asian, Middle East, and African descent are affected (1). Regular blood transfusions are necessary for long-term survival of thalassemia major patients. The iron overload due to chronic blood transfusion adversely affects the function of the heart, liver, and other organs. Iron chelating agents such as deferoxamine, deferasirox, and deferiprone are used to decrease tissue iron concentration (2). The chelation therapy with deferoxamine needs a continuous subcutaneous infusion. The longer infusion period causes fewer side effects and can be more efficient. However, the size and weight of conventional infusion pump (about 25 × 55 × 165 mm3 and 200 grams see Figure 1 for instance) make it difficult for the thalassemia patients to use daily in the long term. Therefore, designing a novel infusion pump with considering reduction on the size and weight is very important to reduce the burden for thalassemia patients. This can allow long-term infusions during the day and night, which ultimately leads to increased quality as well as longer patient life due to the level of the drug in the blood remaining in the therapeutic window during the day.
Micropumps with characteristics such as small size, low weight, and well-controlled on the volume flow rate have been considered a promising candidate for being used as drug infusion pumps. Different micropumps have been developed and categorized for different applications. One of the main parameters of designing a micropump is its actuation method. This is very important in drug delivery systems and biomedical applications. Some micropumps that have been developed for biomedical applications and are suitable for pumping saline solutions are summarized in Table 1.
Since the drug delivery systems are applied to human bodies, some inevitable limitations must be considered in their designing, such as bio-compatibility, high reliability, lifetime, and life safety. For instance, driving voltage should be limited to less than 10 v (3). Thus some of the micropumps that meet the limitation and criteria (lower working voltage with suitable output flow rate; higher than 10 μlit/min) for the present purpose are considered.
| Type | Actuation Mechanism of Micropump | Reference | Voltage (V) | Maximum Infusion Rate (μL/min) |
|---|---|---|---|---|
| Mechanical | Electrostatic | Bourouina et al. (4) | 10 | 0.1 |
| Teymoori and Abbaspour-Sani (5) | 23 | 9.1 | ||
| Zengerle et al. (6) | 200 | 850 | ||
| Piezoelectric | Johari et al. (7) | 16 | 0.0048 | |
| Cazorla et al. (8) | 24 | 3.5 | ||
| Geipel et al. (9) | 100 | 4.5 | ||
| Maillefer et al. (10) | 110 | 13.3 | ||
| Ma et al. (11) | 67.2 | 1800 | ||
| Junwu et al. (12) | 50 | 3500 | ||
| Thermo-pneumatic | Hamid et al. (13) | 10 | 0.0125 | |
| Hwang et al. (14) | 20 | 3.3 | ||
| Jeong et al. (15) | 20 | 21.6 | ||
| Shape Memory Alloy (SMA) | Guo and Fukuda (16) | 6 | 700 | |
| Non-mechanical | Electro-osmosis-DC | Tawfik and Diez (17) | 10 | 80 |
| Electro-chemical | Kabata (18) | 1.4 | 13.8 | |
| AC-MHD | Mastrangelo et al. (19) | 15 | 1900 | |
| Lemoff and Lee (20) | 6.6 | 18 | ||
| DC-MHD | Jang and Lee (21) | 32 | 63 | |
| Huang et al. (22) | 14 | 1114 | ||
| Homsy et al. (23) | 19 | 1.5 | ||
| Nguyen and Kassegne (24) | 5 | 325 | ||
| Lim and Choi (25) | n/r | 0.3 |
Guo and Fukuda (16) proposed a new model of a micropump for pumping microflow rate for medical purposes using SMA coil actuator with 6 v and 0.25 A power supply. The overall size of their micropump is 16 mm in diameter, and 74 mm in length, and the micropump can produce 500 - 700 μL/min flow rate by changing frequency. Kabata (18) developed a micro insulin injection system based on an electrochemical principle. Diaphragm deformed by growing hydrogen bubbles from chemical reactions of electrolyte on platinum electrodes then this exerted pressure to the insulin solution and pumping the liquid into body by a microneedle. The infusion rate could be controlled by electrode potential. Dimension of their micropump was 10 × 12 mm, and maximum flow rate was 13.8 μL/min. Lemoff and Lee (20) proposed a novel micropump using a magnetohydrodynamic (MHD) propulsion system with AC source for generating an electrical field, which has no moving part and produced continuous flow. Their micropump produced about 18.3 μL/min flow rate for 1M NaCl solution, and the dimension of the MHD package is about 50 × 50 mm except power supply dimensions. Nguyen and Kassegne (24) suggested a novel design for DC-MHD micropump with electrodes located inside a reservoir and no moving part, capable of generating a continuous flow in any ionic fluid. The bubble generated in the reservoir cannot enter through the main channel due to bubble isolation and release system located at the upper side of the micropump. The working fluid was a salt aqueous solution, and output flow rate and driving voltage were 325 μL/min and 5 volts, respectively, with 5,000 A/m2 for current density in the main channel.
But these works have some drawbacks that may not be suitable for our purposes: Guo and Fukuda (16) micropump has good response and safety in the body as an implantable device but uses a mechanical mechanism for pumping for which the risk of system failure is considerable in continuous and long-term infusion, which is unfavorable for the use of micropumps in medical applications. Also, working flow rates of the pump are much higher than the required flow rate in our application (about 15 - 25 μL/min), and it is hard to control the infusion rate in low infusion rates. On the other hand, the complexity of their design may lead to not meeting the aim of lowering the cost of the product. Another important drawback of their micropump is high power consumption of the device, which can be considered a significant problem in such pumps. Kabata’s (18) micropump has some significant advantages such as the ability to connect to microneedles, smooth injection without ripples, and controlled injection but the major drawback of their work is the electrochemical reactions that occur on electrodes. This may cause some changes in the therapeutic properties of drug solutions. Also, it is designed for short-time injection (below 300 seconds) and is not capable of continuous infusions. Another drawback of their work is the complexity of the micropump structure and mechanical mechanism that disadvantages of which are described earlier. Lemoff and Lee’s (20) micropump has no moving part then it reduces the risk of performance failure, also has the ability of infusion continuously but needs high power (as much as 2 W) for running. It significantly increases the size and weight of the micropump. Also, as the electromagnet with a voltage amplitude of 23.5 V and the current amplitude of 189 mA are used, the required voltage is higher than safe value (< 10 V) for biomedical applications in contact with human body. Nguyen and Kassegne’s (24) micropump was designed for lab on chip applications and had precise control of fluid flow. Their micropump has no mechanical part then it has lower risk of failure. But the design has complexity in structure, and they did not report capability of long-term continuous infusion for the micropump. The bubble generation during the operation interrupts long-term continuous drug solution flow. Moreover, bubble generation means electrolysis occurs, and as above-mentioned, this can lead to cause some changes in the therapeutic properties of drug solutions.
Therefore, in the present work, a drug delivery system was designed for continuous and long-term subcutaneous infusion with lower physical size and weight for comfort in use. Furthermore, less fabrication complexity for higher availability, non-mechanical part for lower risk of failure, lower driving voltage, and power consumption, and no reaction production in order not to change the therapeutic properties of the drug solution should be considered.
As MHD micropump has a non-mechanical actuation method, then it has some advantages compared to mechanical actuation pumps such as continuous flow forces, simple fabrication process, better reliability and lifetime, low operating failure, and less susceptibility to clogging. Therefore, MHD actuation method is considered for micropump of the drug delivery system. However, bubble generation due to electrolysis of saline solution is one of the major problems in such micropumps.
2. Objectives
This study presents new MHD micropumps for deferoxamine infusion taking into account drawbacks of conventional MHD micropumps such as bubble generation, high weight, and large size.
3. Methods
3.1. Problem Statement
The daily dose required for subcutaneous infusion is about 20 - 40 mg per kilogram of patient weight (maximum five vials), which should be injected at least 8 to 24 hours, five to seven nights a week. It should be noted that the more dilute the solution and the longer the infusion, the greater the effect of the drug on the patient. However, with the conventional infusion pumps, usually five to six times a week for less than eight hours of infusion is performed due to the difficulty that the conventional device imposes on the patient. The present work aims to design and fabricate a drug delivery system that has the ability to store and release a saline solution with a particular infusion rate throughout the day and night. The infusion time is considered to be about 24 hours, and different skin hydraulic resistance is taken into account for an infusion rate of 15 to 25 microliters per minute.
The designed micropump has multiple channels with a rectangular cross-section and certain width (w) and height (h). Electrodes are located at sidewalls; so the distance between them is w (Figure 2). Electrodes are connected to a DC voltage source (V0), which produced electrical field (j). Also, a neodymium permanent magnet with a certain flux density is selected to generate the magnetic field (B0).
The schematic of the considered MHD micropump is shown in Figure 3. It should be mentioned that a mathematical model of the MHD micropump was comprehensively optimized by using NSGAII algorithm (26) to find the suitable configuration and obtain necessary details of the considered design.
3.2. MHD Micropump Fabrication
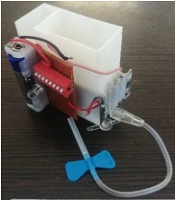
A three-dimensional model of the optimized MHD micropump is generated with CAD for 3D printing by using stereolithography (SLA) technology. This technology has many advantages, such as fast fabrication, high accuracy and precision, isotropic and watertight prototypes, and parts with fine features and smooth surface finish. SLA is a type of additive manufacturing that uses photochemical techniques and concentrated UV light to form the shape of an object, layer by layer. The material which is used to manufacture the device is bio-compatible resin. Figure 4 shows the image of the fabricated micropump.
As seen, the device consists of four integrated parts: reservoir, micropump, collector, and magnet, which were made with a biocompatible resin (with ± 0.15 mm accuracy). Electrodes, which were made from stainless steel and the neodymium permanent magnet are placed into their slots. Then electrodes connected to an electronic circuit consist of DC voltage battery, electric resistances, on/off key, and a dip switch. Reservoir filled with the drug solution from the inlet section. MHD micropump then pumps the drug solution to the outlet via the micropump channels. The outlet is connected to the connecting tube and scalp vein.
A 1.2-volt rechargeable battery and a neodymium permanent magnet with dimension 40 × 20 × 10 mm and magnetic field intensity of 0.2 tesla are attached to the system. Dimensions of the final designed MHD micropump are 20 × 40 × 50 mm3 and the weight of the micropump is about 90 grams. However, it can be smaller and lighter using integrated electrical circuits and a coin battery.
4. Results
First an experimental study is carried out to obtain the hydraulic resistance of skin during the subcutaneous infusion. The experiment was performed by subcutaneous infusion on an anesthetized laboratory rat (Figure 5). A solution of 0.6 cc of ketamine solution was used to anesthetize the live rat, which was injected into the abdomen of the rat, and the specimen was anesthetized for about 45 minutes.
To show the ability and performance of the MHD micropump for pumping in different positions of body with different total hydraulic resistance of skin, Figure 6 is presented. As seen in Figure 6A, with increasing the total resistance, the actuating pressure must be increased in order to function the system properly. The corresponding infusion rate is shown in Figure 6B. This figure clearly showed that the system could deliver proper drugs by easily controlling the actuating pressure (via the Lorentz force).
The experimental setup for studying the drug delivery system performance is represented in Figure 7. As seen, the output of the system is connected to a connecting tube and scalp vein for infusion of the drug solution to the anesthetized rat.
To evaluate the function of the designed infusion pump, 4 cc of deferoxamine was intradermal injected at the maximum infusion rate of the pump on anesthetized rats. Figure 8 shows the time variation of infusion rate for given conditions. As seen, the infusion rate slightly decreases because of changes in the hydrostatic pressure. However, it can easily be compensated with the Lorentz force to maintain the infusion rate constant. Therefore, the results show that the designed pump can inject drug solution into the body at an average injection rate above 20 μL/min (1.2 cc/h).
As mentioned, the amount of drug solution required will vary according to the patient’s weight, so the variability of the infusion rate of the device for injection in different conditions is important.
Figure 9 is presented to show the ability of the designed micropump to infusion at different drug infusion rates. For instance, only by easily changing the external load of the micropump, an average of about 17 µL/min (1 cc/min) (Figure 9A) or about 13 µL/min (0.8 cc/min) (Figure 9B) can be injected.
5. Conclusion
An MHD micropump for drug solution infusion is designed and its performance is presented. The designed optimized micropump is fabricated using three-dimensional printing methods. Bio-compatible resin is selected for micropump fabrication. The performance of the micropump was investigated on the anesthetized laboratory rat. The presented micropump operates with a 1.2-volt rechargeable battery, while its dimension is 20 × 40 × 50 mm3 (about 1/3 conventional micropump size), and its weight is about 90 grams (about half of the conventional micropump). This significant reduction in weight and size of the new infusion pump can allow the patient to use it throughout the day, which leads to greater control of the drug level in the patient’s blood. Also, the infusion micropump can easily produce different actuating pressure to well operate on different skin or patients with different weights. It is shown that the micropump can easily deliver the necessary infusion rate corresponding to different patients’ situations. The micropump overcomes the drawback of the conventional micropump, such as high operating voltages, bubble generation, its large size, and high weight.