1. Background
Autism spectrum disorder (ASD) is a developmental disorder determined by reduced social interactions, limited and repetitive behaviors, decreased eye contact, and delay in speech beginning before 3 years of age (1). The symptoms of ASD are usually manifested at 12 - 15 months of age with a formal diagnosis (2). Standard screening for the diagnosis of ASD was recommended with the age range of 18 - 24 months (2). By providing early intervention services to children with ASD, health policymakers and providers can increase improvement in the dimensions of social, communicative, adaptive, and cognitive outcomes (3, 4).
International prevalence rates for ASD in different countries are variable and have varied widely in recent years. The prevalence rate for ASD in the USA in 2010 was reported as 1 in 69 children (1). In Iran, only a study was published about the prevalence rate of ASD reported as 95.2 per 10,000 individuals (5).
Regarding the increase in the ASD prevalence rate and lower inheritance factor in recent years, it was proposed that environmental factors might play the main role in ASD etiology (6). There were numerous risk factors associated with ASD, including prenatal and perinatal factors, socioeconomic status, and drugs and toxic exposure (7). Among prenatal and perinatal factors, ASD might be associated with maternal infections and preeclampsia in pregnancy, preterm labor, and gestational diabetes (8, 9).
2. Objectives
The identification of the potential risk factors of ASD in large sample sizes is essential to have a comprehensive conception of the nature of the disease. Moreover, adequate motivation for researchers will be provided by the registry to perform various studies in the fields of medical and neuropsychological sciences. Up to the present, no registry system for ASD has been developed in Iran. Therefore, the current study aimed at the generation of the protocol to develop an informative system of ASD registry in Hamadan, one of the western cities of Iran.
3. Methods
3.1. Study Design
The present investigation was a surveillance study developing a protocol for inscribing information about ASD in Hamadan. The approval for this study was obtained from the Ethics Committee of Hamadan University of Medical Sciences (ethics code: IR.UMSHA.REC.1396.485). The parents of children with ASD voluntarily entered this study with written consent. The researchers will explain the confidentiality of the data, the planning of the registry, the purpose of the registry, and the noninterference of the registration process with treatment for patients.
3.2. Case Definition
The autism spectrum is a neurodevelopmental disorder and a variety of mental disorders known as ASD. It contains autism and Asperger syndrome. Individuals diagnosed with ASD frequently show problems with social interaction and communication skills and disruptive behaviors, such as restricted, repetitive patterns of behavior, interests, or activities. The aforementioned symptoms are typically recognized within 1 - 2 years of age (4).
3.3. Eligibility Criteria
The 18-month children will be included in the present study definitively detected in the ASD screening program.
3.4. Data Collection Procedure
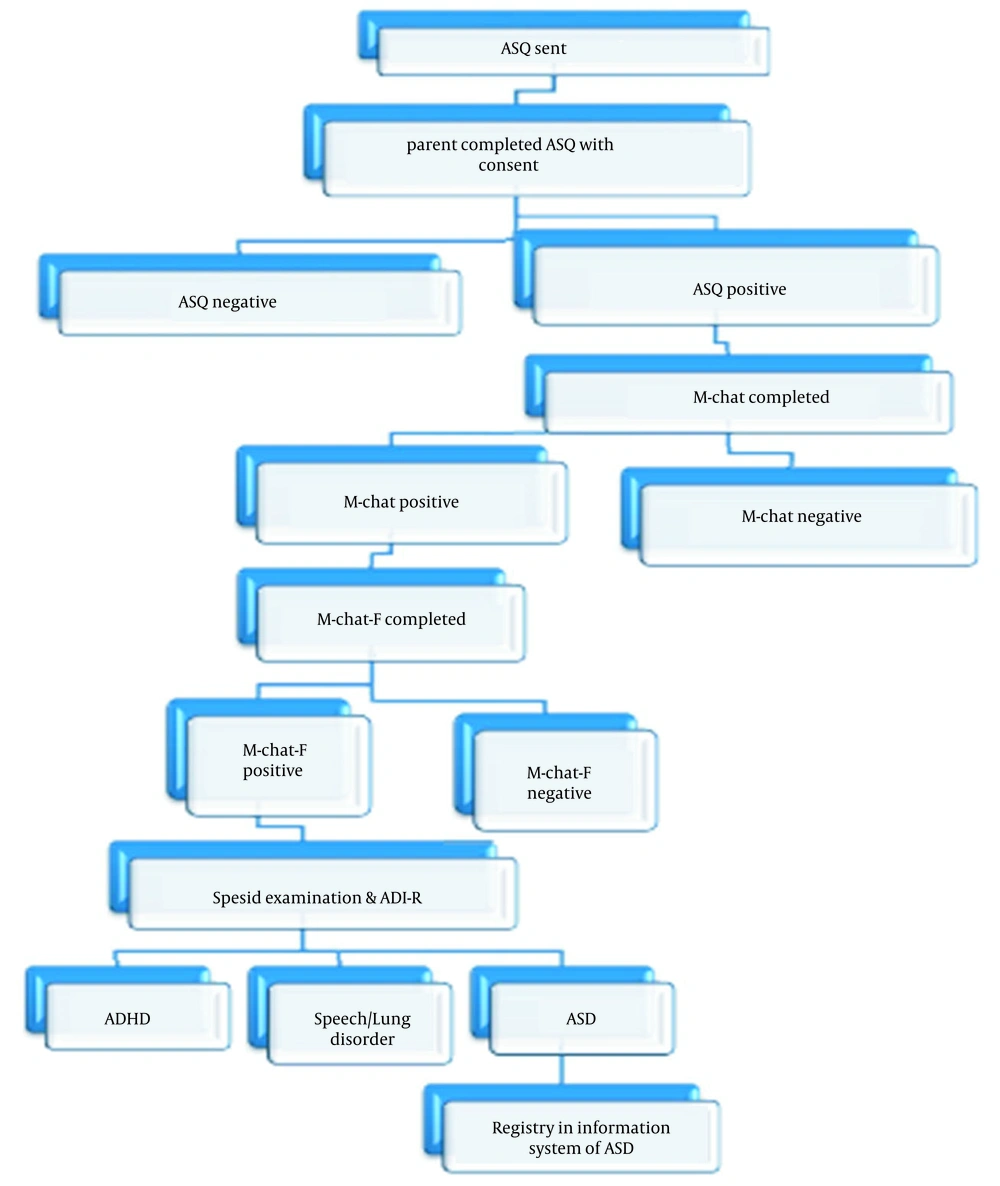
Before designing this protocol, the authors performed ASD screening for children of 18 months. In screening, children of 18 months were included in the SIB system, and the Ages and Stages Questionnaire 18 (ASQ-18) was mailed via a mobile-based application to all children’s parents in the SIB system. The ASQ-18 was filled out by one of the parents. If the child’s score was below the cutoff, the Modified Checklist for Autism in Toddlers (M-CHAT) questionnaire was mailed via a mobile-based application. For those with positive M-CHAT, the formal M-CHAT Follow-Up Interview was performed. The diagnosis of ASD was made by a trained group, such as a pediatrician, pediatric psychiatrist, psychologist, advanced practice nurse, and rehabilitation expertise. The evaluations included a clinical interview of the child, the evaluation of family medical history, observation, and the evaluation of the Autism Diagnosis Interview-Revised. In this way, children of 18 months diagnosed with ASD in the ASD screening will be included in the registry program of ASD. Then, one of the parents of ASD children will reply to questions, and simultaneous data will enter into the web page by a trained expert (Figure 1).
In this web-based interview, it is necessary to respond to all fields of the questions; otherwise, it is not possible to store information in the database. Therefore, missing information will not occur in this web-based interview.
3.5. Measurement Tool
A researcher-made questionnaire will be used for data collection. The questionnaire was reviewed by a strategic team, such as a pediatrician, pediatric psychiatrist, psychologist, reproductive health and rehabilitation expertise, to ensure its validity. For comparability of results, this study used International Classification of Diseases (ICD-10) codes for the types of diagnosis of ASD (e.g., autism, Rett syndrome, and Asperger syndrome). A data dictionary, including the name, definition, and suitable range and values for each variable, is provided to ensure the uniformity of information. The titles of questions in the questionnaire were as follows:
(1) Demographic characteristics of the child and parents
(2) Pregnancy and delivery history
(3) Child development process: Linguistic growth, adaptive skills, talking, communication with others, imitation, reply to the voice and talk of others, visual response, and emotional response
(4) Medical history
(5) Types of diagnosis and clinical tests
(6) Family history
3.6. Quality Assessment
3.6.1. Quality Assurance
The following stages were conducted to ensure the data quality:
(1) Using focus groups and discussions with a strategic team
(2) Holding sessions and workshops and designing ASD children registry software
(3) Increasing Kappa agreement between interviewers by holding workshops
3.6.2. Quality Control
The Data Management website will keep under review all accomplished questionnaires through the registration process. In the case of a defect, a questionnaire will be sent for correction, and the repeated questionnaires will be deleted. The Data Management website will randomly review the data, and some questions might refer to the patient for more confidence.
Randomly each week, a number of patients’ questionnaires registered by the registration expert will be reviewed by the program’s executive director to ensure that data entry is adapted to the software and the questionnaire. If measures are performed, and no difficulties are identified, then the dataset will be prepared for analysis.
4. Results and Discussion
The present study has been funded by Hamadan University of Medical Sciences and obtained ethical approval. Furthermore, the research is ongoing and will be anticipated to be finished by 2021. Therefore, the present study outcomes will be assessed in future studies. In Hamadan, similar to other parts of Iran and on a larger scale worldwide, the number of children diagnosed with ASD is increasing. However, the reasons for this increase are not recognized, and there are no reliable statistics on the prevalence of this disorder in the province to estimate the required resources and facilities for this problem. The determination of the number of children with ASD will allow the researchers to better understand the extent of autism in Hamadan. The understanding of the burden of autism helps design a plan to provide required services for children and families affected by autism. Therefore, the necessity of designing the registration system for autism in Hamadan was felt. Registries also provide more desirable insights into the disease.
The ASD is not integrated into the noncommunicable disease surveillance system of Iran, and there is no routine and population-wide screening program for early diagnosis. Therefore, there is no available reliable data on this topic. The vast majority of prevalence studies regarding ASD were conducted in developed countries (6, 10). However, there is limited information on the identification of children with this condition in developing countries (11, 12).
The use of longitudinal population-based data in registries can help assess time trends in both autism-reported diagnoses and risk factors and evaluate trends in the characteristics of affected individuals over the life course (13). It seems that designing studies related to the prevalence of ASD in different societies has several uses. Firstly, it can alert health policymakers to extend education and provide other required services for ASD children and their families. Secondly, the comparisons of the prevalence of ASD in different societies can show the role of cultural influences and etiological factors (6, 14).
Registries are potent instruments to support healthcare and research and are usually developed based on the data collected from reports or items that is listed by specialists in daily healthcare and cannot be achieved in another way (15). Given the lack of valid and reliable data in less advanced countries in this regard, it seems that the establishment of an ASD registry in these societies with a broad population coverage is essential.
The developed registry system is a precious surveying implement for ASD to have an accurate insight into the fundamental nature of the problem. The essential services and facilities will be provided for children with ASD and their affected families through the obtained information from this registry system. In addition, the ASD registry can provide data from a large population to discover environmental and genetic contributions to the etiological factors of ASD.