1. Background
Pulmonary hypertension (PH) is a condition presenting with abnormal elevation in the pulmonary circulation pressure, affecting the arteries in the lungs and the right side of the heart (1). This progressive disease has a poor prognosis and high morbidity and mortality rates, despite the available therapeutic options (2). Primary PH is a rarely occurring disease that annually affects 4-6 per million people worldwide and four per million people in the United States. Based on the statistics, PH accounts for about140 deaths per year in the United States (3).
The mean pulmonary artery pressure (mPAP) lower than 20.6 mmHg is considered a normal level (4). The gold standard approach for evaluating PH is the right heart catheterization; however, it is an invasive procedure. Based on this approach, an mPAP increase of ≥ 25 mmHg at rest is considered PH (5). There are different ways to evaluate PH. However, the majority of standard techniques for evaluating pulmonary hemodynamics are invasive and impractical for serial assessment. Due to the advances in the understanding of basic mechanisms, clinical features, and therapeutic procedures, the PH diagnostic approaches have changed over time.
Echocardiography is one important tool for screening, diagnosing, evaluating, and following up on patients with PH. Echocardiography is a non-invasive hemodynamic assessment tool in comparison to cardiac catheterization. This modality properly facilitates the assessment of pulmonary arterial hypertension (PAH), left heart disease, chronic lung disease, chronic thromboembolic PH, pulmonary artery pressure (PAP), left atrial pressure (LAP), and pulmonary vascular resistance (PVR) (6). This tool also enables the systolic and diastolic assessment of the right heart and serial follow-up for hypertensive patients. Furthermore, the advancement of technology has facilitated the measurement of the effects of environmental stress, exercise, and hypoxia on pulmonary circulation by echocardiography (7, 8). The prognostic relevance of the echocardiography of pulmonary circulation and PH is clear. Echocardiography can also be used for the screening of subjects prone to developing PH (9).
However, the complexity of right ventricular (RV) anatomy has complicated the assessment of RV function by conventional approaches (10, 11). Accordingly, the insufficiency of these approaches for the diagnosis of PH has been indicated in a number of studies (6, 12). Pulmonary hypertension is one of the major public health problems in children and adolescents that is accompanied by a high mortality rate. Accordingly, the early diagnosis of this condition is of paramount importance since it facilitates the enhancement of survival chance and improvement of quality of life in the affected patients. The use of non-invasive methods for the diagnosis of PH facilitates a faster initiation of treatment, thereby increasing life expectancy and quality of life in the affected patients and decreasing the rate of morbidity.
2. Objectives
Regarding this, the present study was conducted to assess the importance of echocardiographic parameters in children with primary pulmonary hypertension (PPH) and examine their correlation with cardiac catheterization parameters. The results of our study can help in the early diagnosis of the disease and the identification of the more efficient diagnostic methods in this domain.
3. Methods
This retrospective cross-sectional study was conducted on 20 children with primary PH referred to the Pediatric Cardiology Department of Imam Reza Hospital, affiliated with Mashhad University of Medical Sciences, Mashhad, Iran, from September 2001 to September 2017.
3.1. Inclusion and Exclusion Criteria
The inclusion criteria were an age of < 19 years and an infliction with primary PH. On the other hand, children with inaccessible or incomplete medical records were excluded from the study.
3.2. Study Design
This research was performed on 20 patients who were referred or admitted to the Pediatric Cardiology Department of Imam Reza Hospital due to primary PH from September 2001 to September 2017. The demographic data were collected using a researcher-made checklist, including age upon referral, gender, number of children, family history, parental consanguinity, birth weight, and route of delivery. Moreover, the patients were screened for such clinical symptoms as the presence of dyspnea, syncope, cyanosis, shortness of breath, arrhythmia, heart murmur, chest pain, hepatomegaly, edema, and ascites. Furthermore, the data obtained from chest X-ray (CXR), echocardiography, electrocardiography (ECG), and cardiac catheterization, as well as mortality rate, were recorded in a checklist. In case of the incompleteness of the patients' data in their medical records, their parents were contacted. The study protocol was approved by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (P:9142). All data were recorded in a checklist by a predetermined code to ensure confidentiality.
3.3. Statistical Analysis
The data were analyzed in the IBM-SPSS software (version 16) using frequency and mean. After the calculation of the normality of data, t-test or its nonparametric equivalent test (i.e., Mann-Whitney U test) was employed to analyze the variables. We reported qualitative data with numbers and percentages and quantitative data with average and standard deviation (SD).
The correlation between the variables was calculated using the Pearson and Spearman correlation tests. Linear regression was performed to predict some variables. A P-value less than 0.05 was considered statistically significant.
4. Results
According to the results, the participants had a mean age of 6.49 ± 4.82 years and a birth weight of 3.24 ± 0.69 kg. The other categorical demographic data are shown in Table 1. In addition, Table 2 presents the categorical clinical, CXR, and ECG findings. In the present study, the mean ECG rate was measured at 124.12 ± 21.72. Based on the echocardiographic findings, the mean values of tricuspid regurgitation pressure gradient (TRPG) and peak early diastolic transpulmonary valve pressure gradient (PRPG) were obtained as 76.33 ± 22.8 and 13.18 ± 44.2 mmHg, respectively. Furthermore, all patients had dilated right atrium (RA) and dilated right ventricle (RV). In addition, atrial septal defects (ASD) and patent foramen ovale (PFO) were reported in 31.6% (n = 6) and 68.4% (n = 13) of the patients, respectively.
| Variables | No. (%) |
|---|---|
| Age | |
| 0 - 5 | 10 (50) |
| 6 - 11 | 5 (25) |
| 12 - 18 | 5 (25) |
| Gender | |
| Male | 6 (30) |
| Female | 14 (70) |
| Number of children | |
| < 2 | 12 (60) |
| 3 - 5 | 5 (25) |
| > 5 | 3 (15) |
| Birth weight | |
| < 2.5 | 2 (11.8) |
| 2.5 - 4 | 13 (76.5) |
| > 4 | 2 (11.8) |
| Route of delivery | |
| Natural | 13 (72.2) |
| Cesarean | 5 (27.8) |
| Family history | |
| Yes | 0 (0) |
| No | 20 (100) |
| Parental consanguinity | |
| Yes | 4 (25) |
| No | 12 (75) |
| History of seizures and epilepsy | |
| Yes | 3 (20) |
| No | 12 (80) |
Demographic Data of Children with Primary Pulmonary Hypertension
| Clinical Findings | No. (%) | Chest X-ray Findings | No. (%) |
|---|---|---|---|
| Shortness of breath on exertion | Cardiomegaly | ||
| Yes | 17 (85) | Yes | 10 (62.5) |
| No | 13 (15) | No | 6 (37.5) |
| Shortness of breath | RAE | ||
| Yes | 11 (55) | Yes | 12 (12) |
| No | 9 (45) | No | 4 (25) |
| Syncope | RVH | ||
| Yes | 5 (25) | Yes | 12 (75) |
| No | 15 (75) | No | 4 (25) |
| Cyanosis | Prominent pulmonary conus | ||
| Yes | 13 (65) | Yes | 13 (81.2) |
| No | 7 (35) | No | 3 (18.8) |
| Arrhythmia | ECG | ||
| Yes | 6 (30) | Increased | 9 (56.2) |
| No | 14 (70) | Normal | 5 (31.2) |
| Heart murmur | Decreased | 2 (12.5) | |
| Yes | 15 (83) | Rhythm | |
| No | 5 (16.7) | Sinusoidal | 8 (100) |
| Chest pain | Non-sinusoidal | 0 (0) | |
| Yes | 6 (31.6) | Axis | |
| No | 13 (68.4) | RAD | 7 (87.5) |
| Edema | Normal | 1 (12.5) | |
| Yes | 4 (20) | RAE | |
| No | 16 (80) | Yes | 2 (33.3) |
| Hepatomegaly | No | 4 (66.7) | |
| Yes | 18 (10) | RVH | |
| No | 2 (90) | Yes | 4 (66.7) |
| Ascites | No | 2 (33.3) | |
| Yes | 2 (10) | ST change | |
| No | 18 (90) | Yes | 0 (0) |
| No | 6 (100) |
Clinical Findings, Chest X-ray Findings, and Electrocardiography of Children with Primary Pulmonary Hypertension
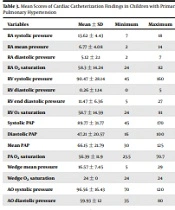
Table 3 shows the mean values of cardiac catheterization findings. Systolic PAP, diastolic PAP, and mPAP to aortic pressure ratios were estimated at 0.92, 0.78, and 0.88, respectively. The right axis deviation, RV hypertrophy, and right atrial engagement were the most common abnormal CXR findings, respectively. Moreover, dilated RV and dilated RA, followed by ASD or PFO, were identified as the most common abnormal ECG findings. The analysis of data showed a significant correlation between systolic PAP and TRPG (r = 0.62; P = 0.008). However, the former variable significantly correlated with PRPG (r = 0.58; P = 0.03). Furthermore, diastolic PAP was found to significantly correlate with TRPG (r = 0.67; P = 0.003) and PRPG (r = 0.64; P = 0.04). Moreover, mean PAP demonstrated a significant association with TRPG (r = 0.66; P = 0.004) and PRPG (r = 0.64; P = 0.04). The results revealed no correlation between the mPAP and demographic information (P > 0.05), except for parental consanguinity (P: 0.04). Moreover, there was no correlation between age and clinical findings (P > 0.05), except for arrhythmia (P: 0.003). In general, 56.3% of the patients passed away. The results showed no significant difference between the dead and survived patients in terms of the correlation between echocardiographic and cardiac catheterization findings (P > 0.05).
| Variables | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| RA systolic pressure | 13.62 ± 4.43 | 7 | 18 |
| RA mean pressure | 6.77 ± 4.08 | 2 | 14 |
| RA diastolic pressure | 5.12 ± 2.1 | 2 | 7 |
| RA O2 saturation | 58.3 ± 14.24 | 24 | 82 |
| RV systolic pressure | 90.47 ± 28.14 | 45 | 160 |
| RV diastolic pressure | 0.26 ± 1.14 | 0 | 5 |
| RV end diastolic pressure | 11.47 ± 6.36 | 5 | 27 |
| RV O2 saturation | 58.7 ± 14.59 | 24 | 81 |
| Systolic PAP | 89.77 ± 31.77 | 45 | 170 |
| Diastolic PAP | 47.21 ± 20.57 | 16 | 100 |
| Mean PAP | 66.15 ± 21.79 | 30 | 125 |
| PA O2 saturation | 56.39 ± 11.9 | 23.5 | 70.7 |
| Wedge mean pressure | 16.57 ± 7.45 | 5 | 29 |
| Wedge O2 saturation | 24 ± 0 | 24 | 24 |
| AO systolic pressure | 96.56 ± 16.43 | 70 | 120 |
| AO diastolic pressure | 59.93 ± 12 | 35 | 80 |
| AO mean pressure | 75.12 ± 13.35 | 55 | 100 |
| AO O2 saturation | 88.15 ± 13.78 | 48 | 100 |
Mean Scores of Cardiac Catheterization Findings in Children with Primary Pulmonary Hypertension
By performing two-variable linear regression to predict the dependent variable PI from the independent variable Peak Pulmonary Atrial Pressure (PAP), the following formula was obtained: PI = 28.004 + 0.346 PAP. Following each increase in PAP, the PI increases by 0.35 units, and if the PAP is zero, the PI is expected to be 28 units (P: 0.04). The following formula was obtained to predict the dependent variable PI from the independent variable PAmax: PI = 24, 38 + 0,307 Pamax (P: 0.04). The following formula was obtained to predict the dependent variable Tricuspid regurgitation (TR) from the independent variable PAs and PA max: TR = 36.97 + 0.495 Pas (P: 0.008) TR = 29.78 + 0.776 PA max (P: 0.004). Following each increase in PAP, the PI increases by 0.35 units, and if the PAP is zero, the PI is expected to be 28 units.
5. Discussion
Based on the results of this study, TRPG had a strong correlation with systolic PAP, diastolic PAP, and mPAP. Moreover, PRPG was strongly correlated with diastolic PAP and mPAP but not with systolic PAP. Therefore, ECG can be concluded to be a proper tool for the diagnosis of PH. To the best of our knowledge, mPAP is an important hemodynamic indicator for the detection and management of PH, which is commonly determined by an invasive procedure known as right heart catheterization. Obviously, a non-invasive method capable of estimating mPAP is always preferred, especially for the serial or repeated evaluation of mPAP (13). Although the use of a non-invasive tool is suggested in many guidelines (14, 15), there are insufficient data on the non-invasive quantification of mPAP. Accordingly, few studies have addressed the reliability of echocardiographic examination. There are various techniques for the assessment of left ventricular function. However, they are not applicable to the RV due to their complex geometry, thin wall, and various contraction patterns.
Our results were indicative of a strong correlation between TRPG and mPAP. Similarly, Shiino et al. (13) reported a good correlation between these two variables. However, the correlation obtained between TRPG and negative peak strain (RV-PS) in the mentioned study was not as strong. They concluded RV-PS as a proper indicator for the detection of increased mPAP in patients with chronic thromboembolic PH. Moreover, in the mentioned study, a correlation was observed between TRPG and peak pulmonary artery systolic pressure (PASP). Moreover, a study was conducted by Yin et al. (16) on the accuracy of echocardiography as used to determine an estimate sPAP. They confirmed a strong correlation between sPAP and right ventricular systolic pressure, as measured by catheterization. However, the correlation between sPAP measured by catheterization and sPAP using echocardiography was relatively weak. The accuracy of echocardiography was estimated at 57.5% for sPAP.
However, the findings obtained by Rich et al. (17) were suggestive of the inaccuracy of the echocardiographic estimation of PASP. Therefore, they recommended not to rely on this modality for the establishment of PH diagnosis. Consequently, RV-PS is a more proper maker for the detection of elevated mPAP in comparison to PASP as an echocardiographic parameter. The RV function in the clinical setting is determined by different conventional echocardiographic parameters, like tricuspid annular plane systolic excursion, RV fractional area change, RV index of myocardial performance, and tricuspid annular peak systolic velocity (18). Although the assessment of regional left ventricular function by echocardiography is a common procedure, recently, regional RV function has also been evaluated by this approach (19, 20). Given that systolic longitudinal deformation is determined by peak systolic strain, a reduction of this indicator can reflect RV dysfunction.
In a study carried out by Ikeda et al. (21) peak systolic strain, post-systolic strain index, TRPG, and end-diastolic diameter were correlated with mPAP and PVR. They suggested peak systolic strain as the only independent predictor for mPAP and PVR. Kasai et al. (22) evaluated different echocardiography-derived prediction indices using direct right heart catheterization to identify the most reliable non-invasive indicator of PVR in patients with chronic thromboembolic PH. In the mentioned study, the highest correlation was observed between TRPG, as an echocardiographic parameter, and PVR assessed by the right heart catheterization. Moreover, PVR was also correlated with tricuspid regurgitation (22). The PVR can be predicted by means of echocardiography via various formulas. The right heart catheterization involves the calculation of PVR equation, while echocardiography facilitates the measurement of RV stroke volume (22).
In confirmation of our findings, Greiner et al. (23), investigating a large sample size, showed a strong correlation between mPAP in ECG and cardiac catheterization. Their results were also indicative of the high diagnostic sensitivity and specificity of ECG tool for the diagnosis of PH. However, the results of a cohort study conducted on patients with advanced lung disease showed that half of the patients were false positive for PH as determined by echocardiography. It seems that the factors related to chronic pulmonary disease may have influenced the findings and limited the accurate measurement of the tricuspid regurgitation jet (24). In our study, only one case with pulmonary problems was reported.
The overestimation and underestimation of PAP in the subjects assessed for PH may also suggest the inaccuracy of echocardiography (17, 25, 26). This issue has been assessed in three recent meta-analyses. Based on the obtained results, the mean time interval between echocardiography and right heart catheterization ranged between two hours and 90 days. Although one of the meta-analyses indicated the low accuracy of echocardiographic findings, they all reported a high diagnostic sensitivity for this procedure. However, the calculated specificity varied among the studies (27-29). Wang et al. (30) confirmed high diagnostic sensitivity and specificity of RV systolic pressure in ECG as a diagnostic tool for the detection of PH. On the other hand, Hua et al. (31) reported the high diagnostic value of SPAP, a factor assessed by ECG.
In a study by Kim et al. (32), a strong correlation was found between RV systolic in ECG findings and PA systolic in cardiac catheterization. They also reported high diagnostic sensitivity and specificity for ECG parameters in detecting patients with PH. Moreover, Hammerstingl et al. (33) detected a high correlation between echocardiography and cardiac catheterization findings. Although annual echocardiographic screening is suggested for the early diagnosis and management of PAH (14), the high sensitivity and specificity of this tool for diagnosing PH have not been proven yet (34).
5.1. Research Limitations
Some of the limitations of the current study include the low sample size, incomplete data, and lack of access to some samples due to the retrospective nature of the research. Therefore, it is suggested to perform further multicenter studies with a larger sample size to investigate the reliability and applicability of using echocardiography for the diagnosis of PH in the pediatric population.
5.2. Conclusions
Echocardiographic approach is a safe and sensitive method for diagnosis of primary pulmonary hypertension. According to the results of the present study, there is a strong correlation between mean PAP and two variables of TRPG and PRPG. Moreover, TRPG was found to correlate with systolic and diastolic PAP.