Introduction
First blood vessels in a process known as vascologenesis forms from endothelial progenitor cells with novel specific patterns and gradually began to spread, develop and form new branches, which it called angiogenesis [1]. Formation of new blood vessels in adults is controlled precisely than previous vessels and angiogenesis just occurs in a physiological condition such as pregnancy and specific pathological conditions such as wound healing, diabetic retinopathy, rheumatoid arthritis or cancer. Since angiogenesis happen in pathological condition such as tumor growth and metastasis and plays an important role, it can also be targeted for antitumor therapy. In general, the process of angiogenesis considered to have 10 stages which one or more sequential steps could of is targeted by angiogenesis inhibitors or simulator [2]. This process is extensively dependent interactions between different cells and molecules and also is controlled by a variety of peptides and moderating factors [3]. Several factors, such as VEGF (vascular endothelial growth factor), FGF (fibroblast growth factor), PTGF (platelet derived growth factor) and STAT3 (signal transducer and activator of transcription) are involved in this pathway [4]. One of the most important factors in angiogenesis, is vascular endothelial growth factor which is a major growth factor for endothelial cells and is a member of the PDGF/VEGF growth factor family and encodes a protein that is often found as a disulfide linked homo dimer. This protein is a glycosylated mitogen that specifically acts on endothelial cells and has various effects, including mediating increased vascular permeability, inducing angiogenesis, vasculogenesis and endothelial cell growth, promoting cell migration, and inhibiting apoptosis [5]. Vascular endothelial growth factor receptors have 3 member and belongs to transmembrane tyrosine kinase receptors (RTK) that is mediator of VEGF-induced endothelial proliferation, survival, migration, tubular morphogenesis and sprouting. The signaling and trafficking of this receptor are regulated by multiple factors, including Rab GTPase, P2Y purine nucleotide receptor, integrin α5β3, T-cell protein tyrosine phosphatase, etc. is a major factor in the process of new blood vessel sprouting from the vascular base and is known as a key factor in signaling system. Among these three receptors, VEGFR-2 is generally recognized to have a principal role in mediating VEGF-induced responses. VEGFR-2 is considered as the earliest marker for endothelial cell development and directly regulates tumor angiogenesis [6]. VEGF-A is the major form that binds and signals through VEGFR-2 to develop blood vessels and maintain the vascular network [7, 8]. VEGF binding to VEGFR-2 triggers the specific activation of tyrosine amino acid residues within intra cytoplasmic tail of the receptor inducing multiple signaling networks that result in endothelial cell survival, proliferation, migration, focal adhesion turnover, actin remodeling and vascular permeability. Signaling through mitogen-activated protein kinase and phosphatidylinositol 3' kinase is a common receptor tyrosine kinase activation pattern. Additional signaling pathways are also triggered upon VEGFR-2 activation, i.e., phospholipase C gamma, protein kinase focal adhesion kinase and T cell-specific adapter -Src kinase which are linked to migration and vascular permeability [9, 10]. At in vivo condition VEGF effect on vascular permeability which is essential for angiogenesis, and regulate it, so that is called vascular permeability factor and in this case acts several times more potent than histamine [11]. Under hypoxic conditions, reduction of oxygen pressure is crucial and tissues start synthesis and releases angiogenic factors such as endothelial cell growth factors after binding to their special receptors, activate them leads to secretion of metalloproteinase from endothelial cells to digest and decomposition basement membrane in the region. By reaching this aim endothelial cells would able to migrate and proliferate, in addition, the molecule of adhesion such as integrins α7β5 and α3β7 are helps in the process of pulling forward and bud blood vessels develop, in the next steps in the process of angiogenesis, matrix metallo proteinases (MMP) to degrade extracellular matrix and start re-reconstruction produced. By the interaction of the Tie-2 with angiopoitine tube formation begins at a later stage, EphB-ephrin B also regulates formation the tubes, finally smooth muscle cells and precyts added to inducing the stabilization of newly formed blood vessel structure [12, 13].
Rising of cancer resistance to ordinary therapies, increase effort to find out new era in cancer therapy with fewer side effects and resistance [14]. Since cancer is growing in the world, and each year cause death of more than 6 million people and there is no cancer as concerning as breast cancer which having the highest rates of mortality at ages 40 to 44 years to occur [15, 16]. This cancer is a hormone-dependent disease which many of risk factors are known [17]. Some of these risk factors are: geographic diversity, age, family history, pregnancy history, pregnancy, benign breast disease, exogenous estrogens, oral contraceptive drugs, obesity, high-fat diet, alcohol consumption, smoking and radiation chest out [18, 19]. Breast cancer in Iranian women compared with Western women, occurs at least one decade earlier and in more advanced stages. Its occurrence among Iranian women was 120 per 100,000 women. Interfere with normal function of at least 4 to 6 genes by genetic and epigenetic mechanisms can lead to breast cancer, which disturb the balance between proliferation, apoptosis and differentiation, and also regulation of steroid receptors, binding cells and angiogenesis [20].
Therefore, research and development of more effective drugs with fewer side effects and has become increasingly important in tumor angiogenesis. Due to the side effects of chemical drugs, herbal medicine with minimal side effects and drug interactions are noticed. Meanwhile, saffron, which has a special place in people's nutrition, is important [21]. Saffron (Crocus sativus Linn.) belongs to Iridaceae family with sweet fragrance and bitter taste perennial with purple flowers that have a long three parts orange-red stigma which has commercial value as a most expensive spice [22]. Saffron have been used as a flavor in foods and in the treatment of a vast spectrum of disorders like coughs, flatulence, gastrointestinal disorder, insomnia, chronic uterine bleeding disorders, femininity, scarlet fever (scarlet) and even cancer [23]. It has been demonstrated that its main component such as crcin, crocetin and saffranal can inhibit neurons erosion, and its anti depression [24, 25].
Saffron as a protective agent, prevent damages to chromosomes and also modulating lipid peroxidation and work as a powerful antioxidant and a rich source of riboflavin [26]. Saffron have antioxidant, geno toxic, anti-tumor and cytotoxic properties against cancer cells which is related to its active metabolites (safranal, crocin, picrocrocin, crocetin and dimethyl crocetin and anthocyanin, carotene and lycopene) and these compounds possess the pharmacological effects [27]. It has also recently been reported that the saffron extract has effective anti-cancer properties in different types of leukemia, osteosarcoma, fibro sarcoma, carcinoma [28].
One of the most practical uses of real time PCR is defining gene expression using methods such as quantitative relative which is one of the precise and particular ways to study different changes in gene expression. In this method (Relative Quantitative) reduction or increasing of gene expression is important which is compared with reference gene, this referenced gene is a house keeping gene with specific and individual character such as equal copy number in all cancerous cells, capability of expression in all body cell and not affected by treatment. In this project is not necessary to know the quantity of samples, because it is based on the gene expression of the gene in compare with reference gene [29].
As regards to identification of an effective strategy by using new agents from natural sources to treatment cancers, and since vascular endothelial growth factor and its receptor are potent endothelial mitogen that are up regulated in number of tumor types, including breast cancer, the aim of this study was to investigated the effect of saffron aqueous extract on level of VEGF-A and VEGFR-2 in human breast cancer cell line (MCF-7).
Materials and Methods
Saffron sample and preparation: In the experimental study original Iranian saffron samples were produced in Khorasan. Saffron samples (3 g) were aqueous extracted with Soxhlet. The mixtures transferred to Rotary to remove the water. In order to drying the extract the freezing and in a vacuum drying (lyophilisation) using freeze dryer was used. Cell culture: MCF-7 cells were obtained from Pasteur Institute (Tehran, Iran).
Cells were cultured in RPMI medium (Biosera) with 10% fetal bovine serum (Gibco, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin (Sigma, French) and also L-glutamine 1 mL (Sigma, French ) and incubated at 95% humidity, 5% CO2. After 24 h of culture and insure about cell adhesion to the substrate of flask, cells were treated with aqueous extract of saffron stock 10 μg/μL with concentrations of 100, 200, 400 and 800 µg/mL was. Then to evaluate the viability (viability test) Trypan blue (Sigma, French) test was used and for 5 days shooting with a digital camera with invert microscope (Dinocaptre) (200 X magnification) done.
RNA extraction: RNA extraction done by total RNA purification kit (Jena Bioscience Germany) after 48 h of treatment according to the protocol attached to the kit. Total RNA purified was stored at minus 20ºC until cDNA synthesis. To measure the amount of RNA, extracted products with 2 mL of buffer were measured and analyzed by Nanodrop at wavelengths of 260, 280 and 320 nm. These data indicate the concentration of RNA which was used for cDNA synthesis.
RNA concentration= (OD 260 - OD 320) × 40 × 100
cDNA synthesis: cDNA synthesis by kit Accu Power ® Racket Script TM RT Pre Mix (Korea), and synthesis performed according to kit protocol.
Synthesis of primers: The sequence of genes were received from NCBI site and the bio informatic validation of RT-PCR primers were done in PRIMER BLAST. Primers were purchased according to table 2.
Evaluation of gene expression using real time PCR: Process of real time PCR to study gene expression done according to the protocol by Bioneer kit from South Korea which its brand was Accu Power ® 2 X Green star qPCR Master Mix by Applied Bio systems. According to the protocol Master Mix firstly was prepared and added to strip cap micro tube then the cDNA was added to it.
Application temperature according Tm of designed primers and to the characteristics of the different phases polymerase chain reaction were determined.
Gene expression levels of VEGF-A and VEGFR-2 in samples treated with aqueous extract of saffron in comparison with control samples were analyzed by statistical software SPSS-16. Fisher test p-value of less than 0.05 and 95%CI (Confidence Interval) was accepted as significant. Gene expression levels of VEGF-A after 48 h of incubation of cells with different concentrations of saffron extract was measured. The gene expression levels were compared with a control sample without the influence of extracts. In this project we use relative quantitative which is based on expression of target gene to the reference gene via comparing the target gene efficiency with control sample and also using their Ct. The primers and the temperature of binding primer are essential factors in the optimization of RT PCR reaction. So condition of reaction prepared optimize in which no nonspecific product produced that can easily seen by mono peak in melting curve and also electrophoresis in agar gel by looking single sharp band.
Results
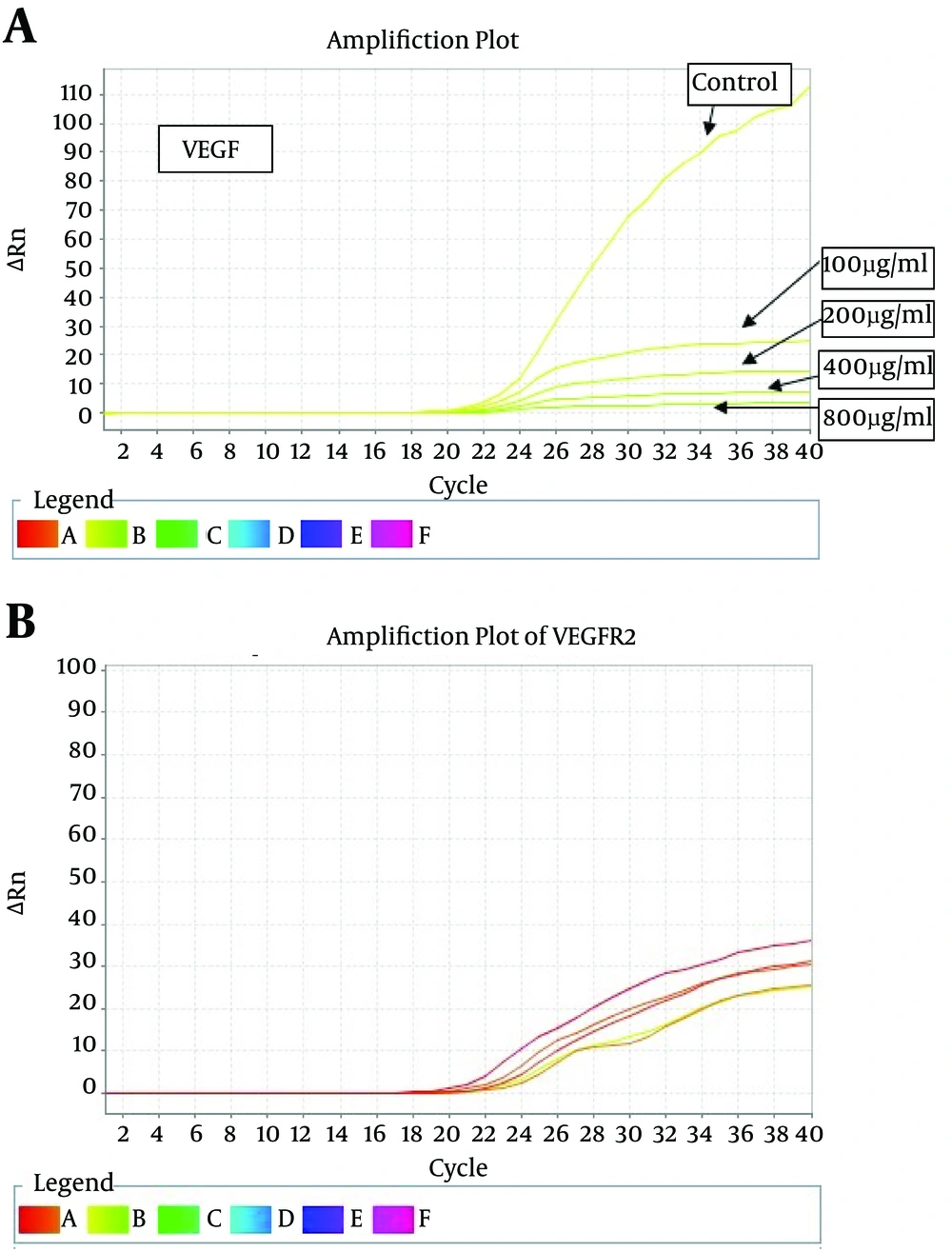
Results of morphologic research of cells with treatment of saffron extract shows dose and time dependence decline and obvious reduction of cytoplasm which was pigmented in the way that in higher concentration and passing the time morphologic changes such as granulation, inhibition of growth, pigmentation and cell consuming was observed. Since the PCR reaction efficiency between the target gene and the housekeeping gene (beta-actin) was the same (Fig. 1, 2), the comparative threshold cycle (TC) was used in this project. Amplification plot of treated with aqueous extract of saffron shown in figure 3.
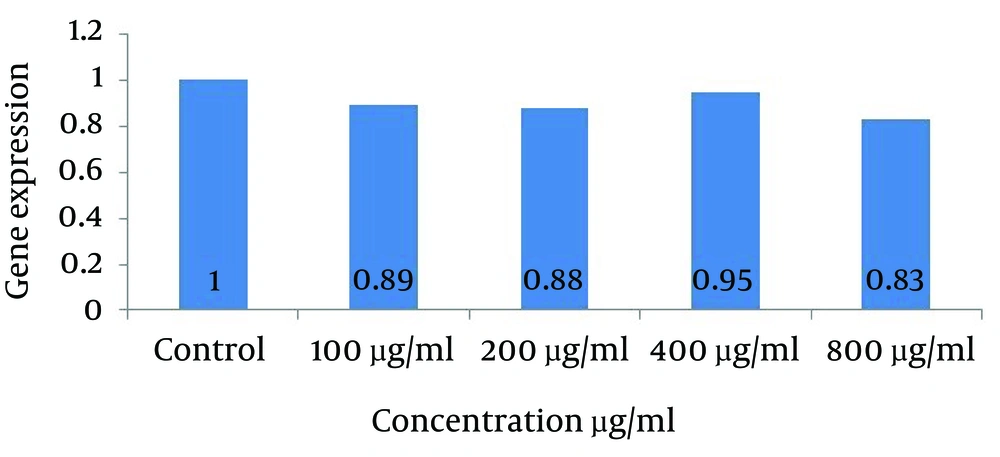
Gene expression studies showed a significant reduction (less than 5%) in the gene expression levels of VEGF-A gene treated with concentrations of 100, 200, 400 and 800 µg/mL of cells comparing with the control group (not treated with the extract). The greatest changes in gene expression were associated with the highest concentration of the extract.
Reduction of vascular endothelial growth factor (VEGF-A) gene expression in groups treated with the extract at concentrations of 100 (0.89) and 200 (0.88) μg/ml shows no significant difference with each other while both of them compared to the control group show reduction at p˂0.05. The decreasing in gene expression in the group treated with aqueous extract of saffron at a concentration of 800 μg/mL (0.83) compared to the control group shows a meaningful decline at p˂0.01. This experimental group shows the most noticeable reduction with 17% decline in VEGF-A gene expression between other groups (Fig. 4).
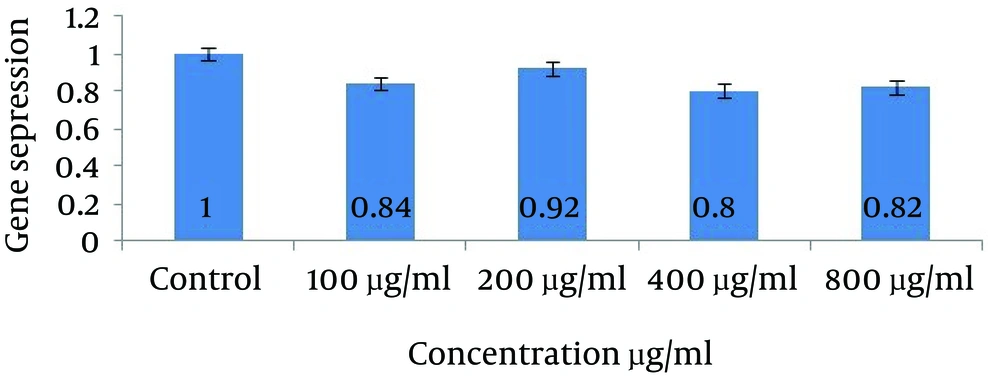
For VEGFR-2 gene expression, significant reduction for concentration of 400 µL/mL (0.80) was observed with 20% decreased in gene expression comparing with no treatment (p˂0.01). This noticeable fall also have been seen in concentration of 100 (0.84) and 800 µg/mL (0.82) tests at p˂0.01 while in second experimental group (200 µg/mL) it was not remarkable (p˂0.05). Data shown in figure 5.
| Step | Temperature | Time |
|---|---|---|
| Primer annealing | Tm of specific primer | 1 min |
| cDNA synthesis | 42-70°C | 10-60 min |
| Heat inactivation | 95°C | 5 min |
| Gene | Forward 5→3 | Reverse 5→3 | TM | Gene ID | Chromosomal location | ACCESSION gene bank |
|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor (21mer) | TAT GTC TAT GTT CAA GAT TAC | AAG TTT CTT ATG CTG ATG CTT | 42/9 | 3791 | 4:q11-q12 | 1356aa NP-002244.1 |
| Vascular endothelial growth factor A (19mer) | CCT GCC TTG CTG CTC TAC C | CAC ACA GGA TGG CTT GAA G | 52/0 | 7427 | 6:p12 | 237aa AAH11177.2 |
| Βeta actin (21mer) | CCC GCC GCC AGC TCA CCA TGG | AAG GTC TCA AAC ATG ATC TGG GTC | 64/5 | 60 | 7:p22 | 109aa ADX779061 |
| Component | Final Concentration (µL) | Dilution (µL) |
|---|---|---|
| 2X Greenstar master mix | 25 | 10 |
| PCR forward primer (10 pM) | 1-2 | 1 |
| PCR reverse primer (10 pM) | 1-2 | 1 |
| 50 X ROX dye | 1 | 0.4 |
| Template | 5-10 | 1 |
| DEPC distilled water | Adjust 50 | 7 |
| Total | 50 | 20 |
| Step | Condition | Cycle |
|---|---|---|
| Pre-denaturation | 95°C, 10-15 min | 1 |
| Denaturation | 95°C, 5-20 sec | 40-45 |
| Annealing/Extension | 55-60°C, 30-45 sec | |
| Detection | Scan | |
| Melting | - | 1 |
Discussion
Therefore it can be concluded saffron extract could reduces angiogenesis in the consumption concentration. So it would be conclude that saffron anti cancer and anti tumor function may be via inhibition of angiogenesis.
Das et al. proposed several mechanisms for the antitumor effect of saffron and its components, including inhibition of nucleic acid, a free radical chain reaction, the effect of carotenoids on the expression of topoisomerase type 2 and induction of programmed cell death [30]. The result of the other research indicate that oral prescription of saffron extract in lab animal could reduce the induced cancer with mutagen and decline the rate of tumor cell growth on one hand and increase the lifelong of animal on the other hand [31]. It is also indicating in some papers that saffron in different cell models on a wide range of tumors, including leukemia, ovarian and breast carcinoma, colorectal adenocarcinoma, rhabdomyo sarcoma, HPV and squamous cell carcinoma have an anticancer effect. While saffron have selective cytotoxic affect. On different malignant cells it has not affect on viability of non malignant and health cells [32]. Results of some research on 2009 reveals that the most important point in curing cancers with synthetic and chemotherapy is the acquisitive resistance of cells to chemical drugs and because mutation and genetic variation in tumor cells are higher than other cells, so they resist to synthetic drugs more. So inhibition of angiogenesis with natural products and herbal plants such as saffron seems to be a useful method in order to encounter less resistance [33]. In addition Mousavi et al. showed that saffron extract has anti tumor effect on HepG2 cell line and notified IC50 for that 400 µg/mL and claimed that anti cancer effect of saffron extract against induced cancers both in vitro and invivo could inhibited and stop some important process such as DNA and RNA synthesis and subsequently cell growth and division and also the inhibitory effect of it on nucleic acid synthesis could be a biochemical basis for its inhibitory effect on cell proliferation is [34]. Moreover other studies also illustrate that saffron has cancer-preventive and anti genotoxic potential and cancer preventive, so it would be used in combination with chemotherapy. Apart from that saffron shows reduction potential against lipid peroxidation and at the same time increase the enzymatic antioxidants like superoxide dismutase and catalase and non-enzymatic antioxidants such as liver glutathione regenerated [35]. Some research results indicate safranal increases tissue oxygen and have sweeping effect on free radicals and can inhibit oxidative stress by genotoxic compounds. Safranal also has protective effect on lipid peroxidation. Since angiogenesis have relation with tissue oxygen and hypoxia is one of the most important motivator of angiogenesis, so increasing in tissue oxygen accompanied with saffron treatment may have define some part of anti angiogenic effect of this herb [36]. Numerous studies have reveals that crocetin is effective on variety of cancers which are curable by retinoic acid and shows that natural cartenoids from saffron such as crocin and crocetin have inhibitory effect on growth and induce differentiation of cells and these components shown effects in leukemia cells [37]. Another study stated that saffron carotenoids and mono-terpene aldehyde have interaction with DNA and could lead to inhibition of growth of colon cancer cells, including HCT116, SW480 and HT29, while there has no observed effect on normal cells [38]. It is also represent that saffron aqueous extract have antitumor effects on TCC cell line (related to bladder cancer) in the time and dose dependent manner in the way that in high concentration percentage of vital cells decline dramatically [39]. These results are consistent with the findings of the present project. Influential potential of saffron on the induction or inhibition of gene expression in few cases has been studied so far. However, Mousavi et al. had studied the effect of saffron extract on the level of protein which were related to apoptosis such as bax protein and enzyme such as caspase in breast cancer cells and represent that using extract could reduce cell viability on one hand dose dependently with the IC50 of 400 μg/mL while there was no effect on normal cells [40]. The experimental results on the induction of apoptosis of this extract depicts that on hepato carcinoma and cervix cells extract has cytotoxic effects and IC50 for the two cell lines HepG2 and HeLa after 48 h, respectively was reported, 950 and 800 μg/mL [41]. The results of the present study were coinciding with these findings and cancer cell growth inhibited by the extract on concentration-dependent manner. In this laboratory research saffron aqueous extract can fall the expression of VEGF-A and VEGFR-2 gene as two important angiogenic biomarker which shows conformity with other data that reveals cytotoxic and anticancer effect of saffron and it is probably that treatment of tumor cells with it inhibit DNA and RNA synthesis and reduce cell growth and angiogenesis.
Since breast cancer is one of the main malignant in the world and many of cancers formed because of the deficiency of biochemical and biological relations on the molecular level and there is less information about mechanism of controlling of cell cycle and growth, so it would be essential and helpful identifying cancer molecular mechanisms and pathways [42]. VEGFs family is a huge family of dependent gene which plays variety of crucial roles in cells such as responsibility for cellular responses like proliferation, migration, and some of developmental pathways like development of branches in kidney ureter bud or pulmonary alveolar, etc. This family member also have variety of stimulatory effects in cancer cells and have ability to activate signaling cascade to formed new blood vessels and because one of the most promising strategy against inhibition of angiogenesis is suppression of VEGFs ligands and could be an effective cure for many malignant [43-45]. Reduction of this family gene expression in vitro could represent part of the anticancer potential of this medicinal plant through anti-angiogenic pathways. More tests to determine the exact mechanisms involved in the anti-cancer potential of saffron is required.
The results of this study indicate a reduction in vitro on VEGF-A and VEGFR-2 gene expression in breast cancer cells treated with the saffron aqueous extract in compare with no treatment cells showed confirms the anti-angiogenic potential of this medical herb.