1. Background
Blood consists of cellular elements, including red blood cells (RBCs), white blood cells (WBCs), platelets (Plt), and plasma. Functions of the blood include transport of gases and nutrients, action as a buffer, stabilization of body temperature, defense against pathogens, and coagulation function (1). Red blood cells comprise 99.9% of blood cells and are one of the most important cellular elements that exchange gases through hemoglobin (Hb) (1). The functions of WBCs and their subclasses, including neutrophils, monocytes, and lymphocytes, are as follows: Production of proteolytic enzymes, phagocytosis of foreign invaders and damaged cells, and synthesis of antibodies against invasive pathogens, respectively (2). Platelets are also the main elements of the blood coagulation cascade, which prevent bleeding and reduction of blood volume by forming clots. In addition to their role in blood coagulation and the regulation of homeostasis, they also contribute to the regulation of inflammatory reactions and immune function (3). Furthermore, the effect of various types of exercise has been shown with different intensities and durations, either as a single session exercise or training period, on blood components (cellular elements and plasma volume) in athletes, non-athletes, and healthy subjects, and patients (4-11). In general, they have presented two approaches: Health goals or championship sports performance goals. In terms of health, they have shown health-threatening disorders due to one session of high-intensity exercise or the health-related benefits of adaptation to a training period on blood components. For example, researchers have examined acute and chronic effects of exercise on RBCs, which affect athlete’s performance directly. For example, Mairbaurl, in their review article, referred to RBC depletion as anemia (exercise-induced anemia) (12). They have shown, conversely, an increase in RBCs, a reduction in their lifespan, and an increase in their deformation due to adaptation to exercise training, which increase O2 transfer capacity in athletes (12). Montero and Lundby have also mentioned the increase in RBCs and plasma volume (PV) and improvement of blood circulation characteristics due to adaptation to exercise training (13). Schumacher et al. also examined RBC counts and iron metabolism in 851 athletes of endurance, strength, and power sports. They showed a lower level of hematocrit (Hct), Hb, and RBCs in endurance athletes in comparison with strength athletes (14). It was further attributed to increased adaptation of PV and was lesser attributed to hemolysis and decreased haptoglobin levels seen only in runners (14). Also, sudden death due to myocardial infarction, which is specifically along with thrombosis due to an increase in Plts, is usually seen in response to a single session of high-intensity exercise. While as reported, adaptation to exercise training decreases blood clotting and increases anti-clotting agents (15, 16). For example, van der Vorm et al., in their review article, demonstrated sudden death and cardiovascular complications after single sessions of high-intensity exercise and attributed them to hemostatic disorders and Plt aggregation (16). Conversely, they reported decreases in blood clotting and increase in anti-clotting agents due to adaptation to exercise training (16). In addition, Simpson et al. have reported temporary weakness of the immune system, and reduction of WBCs due to single session of high-intensity exercise (17). While they have mentioned the increase in these indices due to adaptation to moderate exercise training (17). Although there are many studies about the effects of exercise on hematologic factors, studies on the responses and adaptations in blood components are still ongoing due to the vogue of new sports. CrossFit is a high-intensity interval exercise that has gained a high popularity among sports enthusiasts in recent years (18). However, the high intensity of this exercise has drawn researcher’s attention to the consequences associated with injury and threatening health (18). For example, Larsen and Jensen showed damage and collapse of muscles (rhabdomyolysis) following high-intensity exercise in female athletes (19). Hak et al. also assessed the prevalence of injury during CrossFit training through questionnaires collected from professional athletes (20). They concluded that the prevalence of injury in CrossFit athletes is similar to that in powerlifting and gymnastics athletes and lower than in competitive sports like rugby. Major injuries are related to shoulder and spine injuries, and also, there are cases of rhabdomyolysis (20). Although the short-term effects of CrossFit on the hematological factors have been less considered, studies have been performed on the effect of similar exercises such as high-intensity exercise (HIIE) on these factors. For example, Jamurtas et al. have shown disruptions in WBCs and redox status after HIIT training compared to submaximal aerobic exercise (21). Sheykhlouvand et al. have also shown that a high-intensity boating session increases Plts and WBCs (11). Mairbaurl have also reported RBC hemolysis due to strenuous exercise (12). Therefore, with consideration of high-intensity exercise effects on acute disruption of hematologic factors and with a considerable increase in CrossFit participants, it seems necessary to assess the effects of a single session of CrossFit exercise on Plts, RBCs, WBCs, Hb, Hct, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) in professional male athletes.
2. Methods
2.1. Subjects
In this study, 32 professional male athletes who participated in the semi-final stage of the national CrossFit competition and had a history of functional training and high-intensity exercise training for three to 10 years in the oxygen club of Tehran were selected as subjects. The demographic characteristics of subjects are shown in Table 1. All subjects were healthy, and they had no injury. Informed consent was obtained from all subjects, and all procedures of this study were approved by the Ethics Committee of Sports Science Faculty of Shahid Rajaee Teacher Training University (IR.SRTTU.SSF.2018.117).
| Variable | Mean ± SD |
|---|---|
| Age (y) | 26.9 ± 4.7 |
| Weight (kg) | 80.7 ± 6.4 |
| Height (cm) | 177 ± 7 |
| Body mass index (kg/m2) | 25.6 ± 1.6 |
| Body fat percentage (%) | 9.9 ± 2.7 |
2.2. Procedures
On the day of the competition, the athletes competed in paired groups, and they were in the same situation in terms of motivation. The competition lasted eight minutes, including running for 400 meters, three-stage deadlifting, pull-up (30 repetitions), 30 kg kettlebell swinging, and throwing 20 sand balls with different weights, respectively.
2.3. Blood Sampling and Laboratory Methods
Body composition was identified through bioimpedance analysis (InBody 577, made in South Korea). 4 mL of blood was taken from brachial vein of athletes before and immediately after the competition in a sitting position, and blood was poured into two tubes containing EDTA anticoagulant. All blood samples were stored at a temperature between 3 and 4°C in the competition location and centrifuged for 10 min at 3,000 RPM one hour after the competition (Behdad centrifuge device, made in Iran). The number of cells was measured by cell counter (Sysmex, K1000, US). Biochemistry autoanalyzer also was used to measure lactate (Hitachi911). The levels of Hct and Hb were also used to estimate the percentage change in PV by the Dill and Costill equation (22). Finally, the indices were modified with consideration of the changes in PV, according to the following formula (23):
2.4. Statistical Analysis
The data were presented as the means and standard deviation (mean ± SD). The Kolmogorov-Smirnov test was used to specify the normality of data distribution. ANOVA with repeated measure and Bonferroni post hoc tests were used to examine the differences between variables in resting position, post-exercise, and after correction for the volume of plasma lost. The P-value of less than 0.05 was statistically considered significant.
3. Results
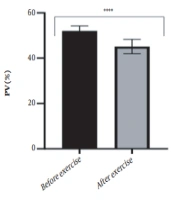
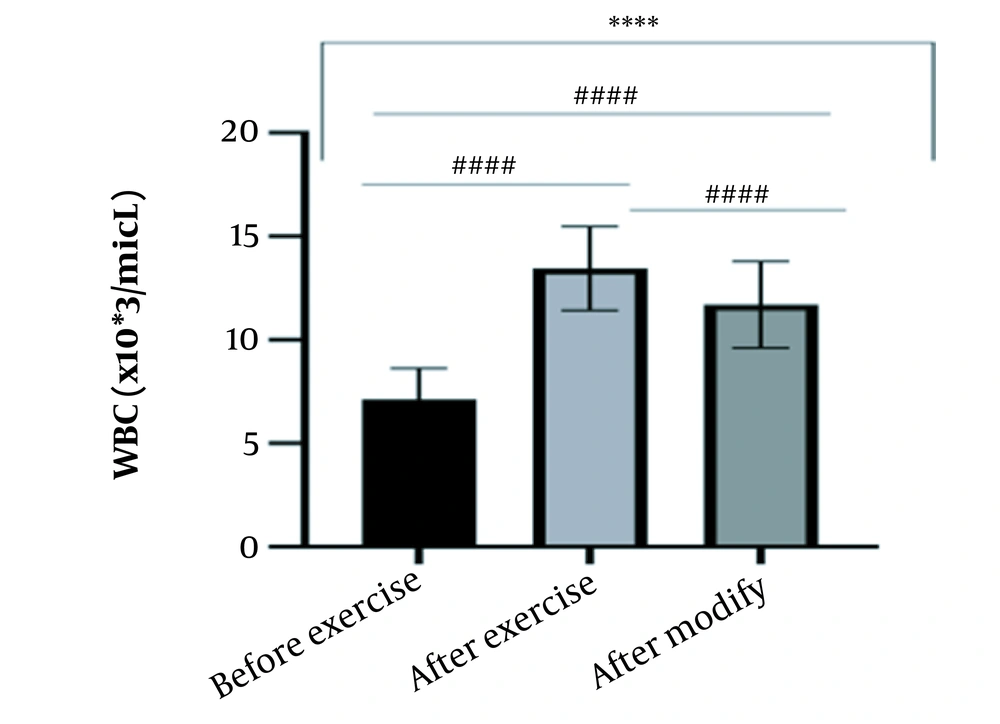
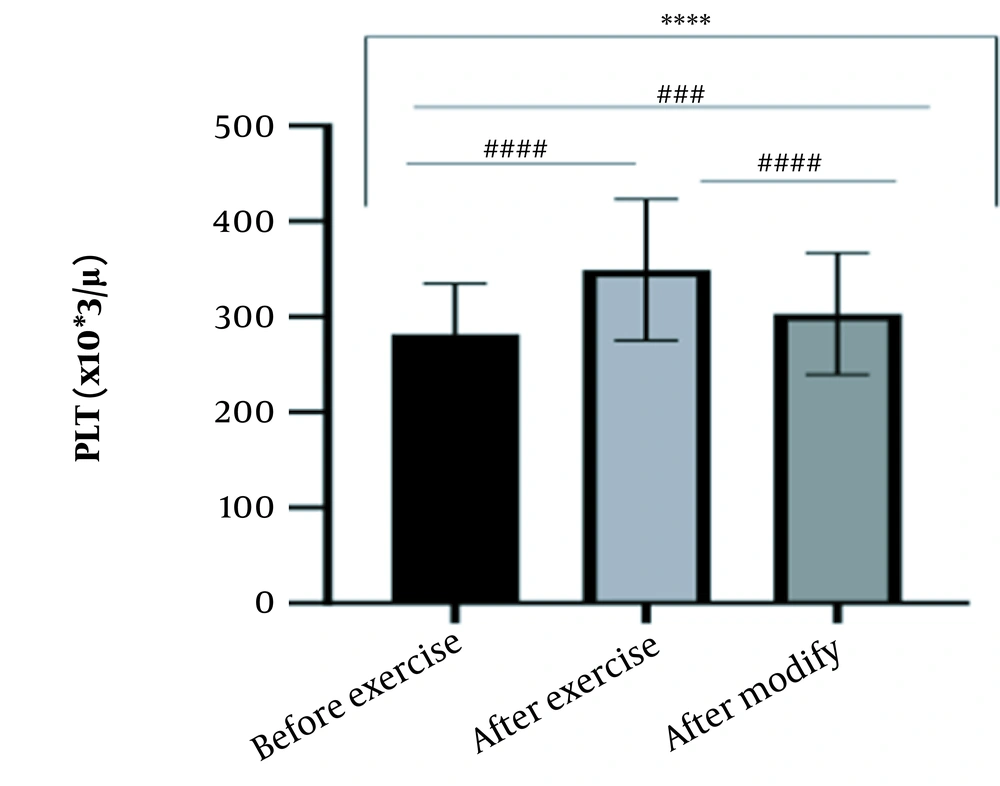
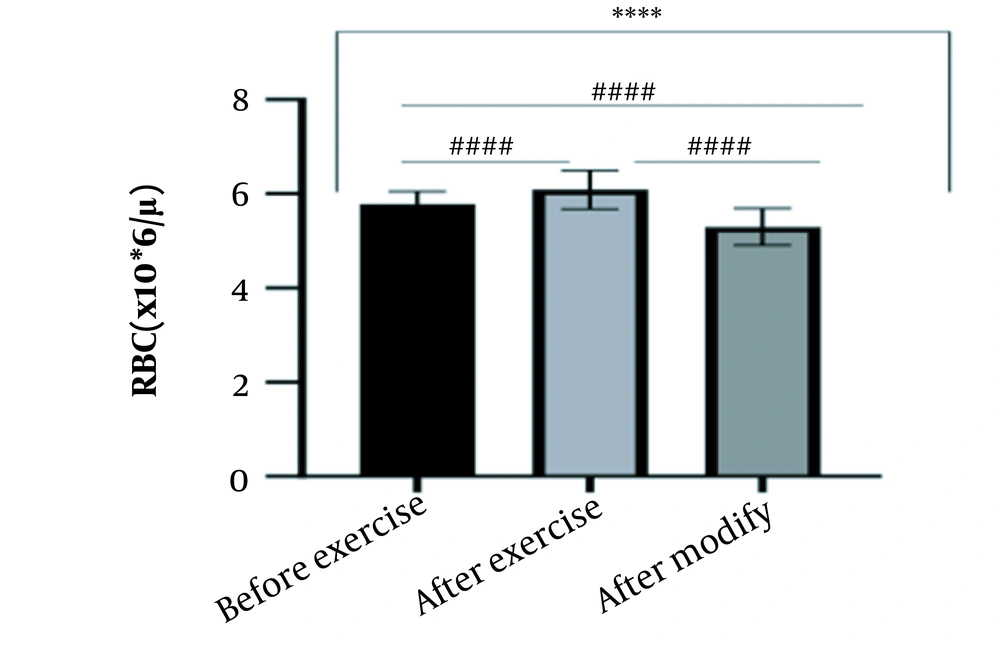
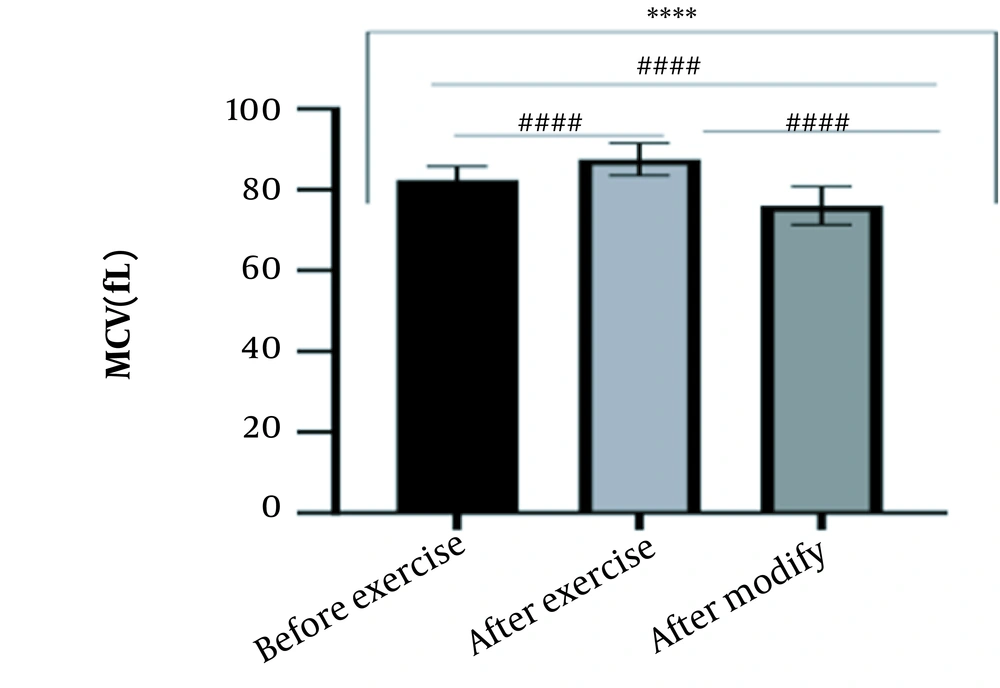
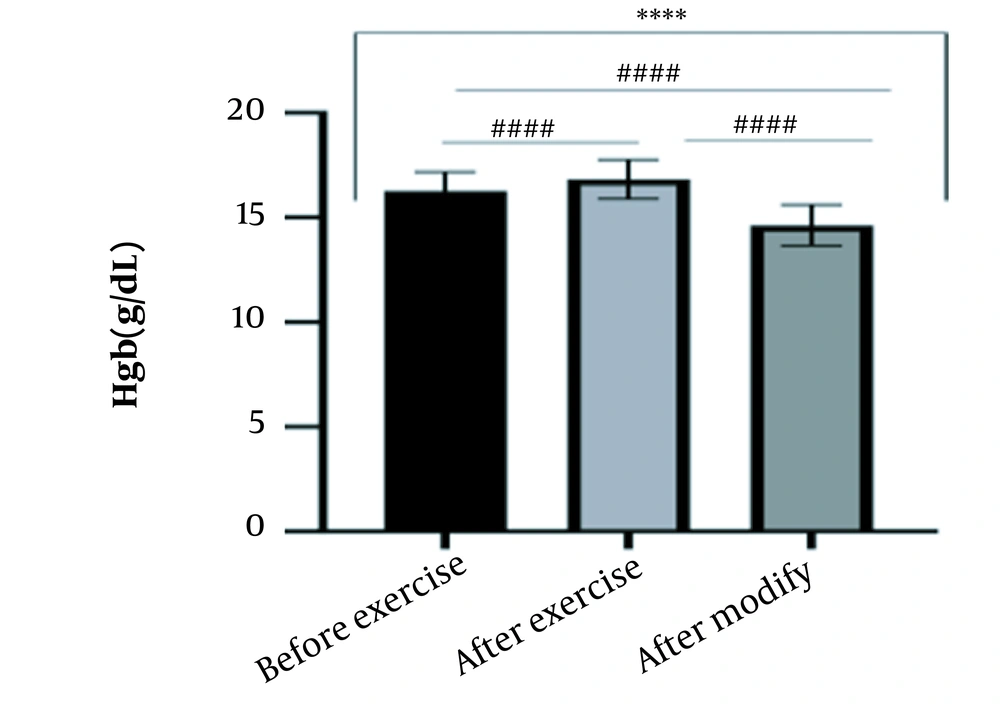
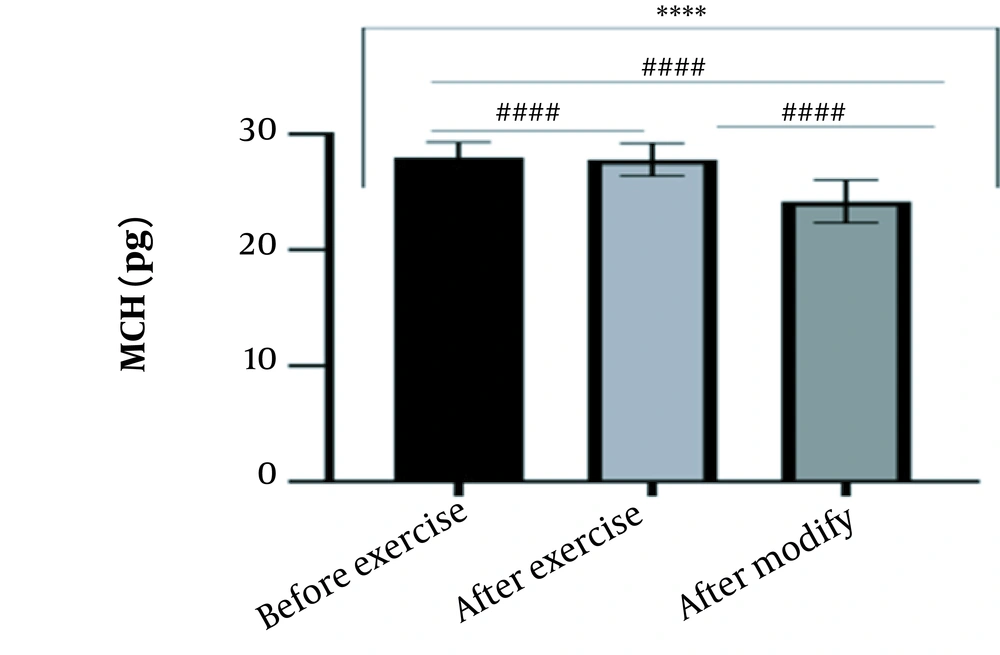
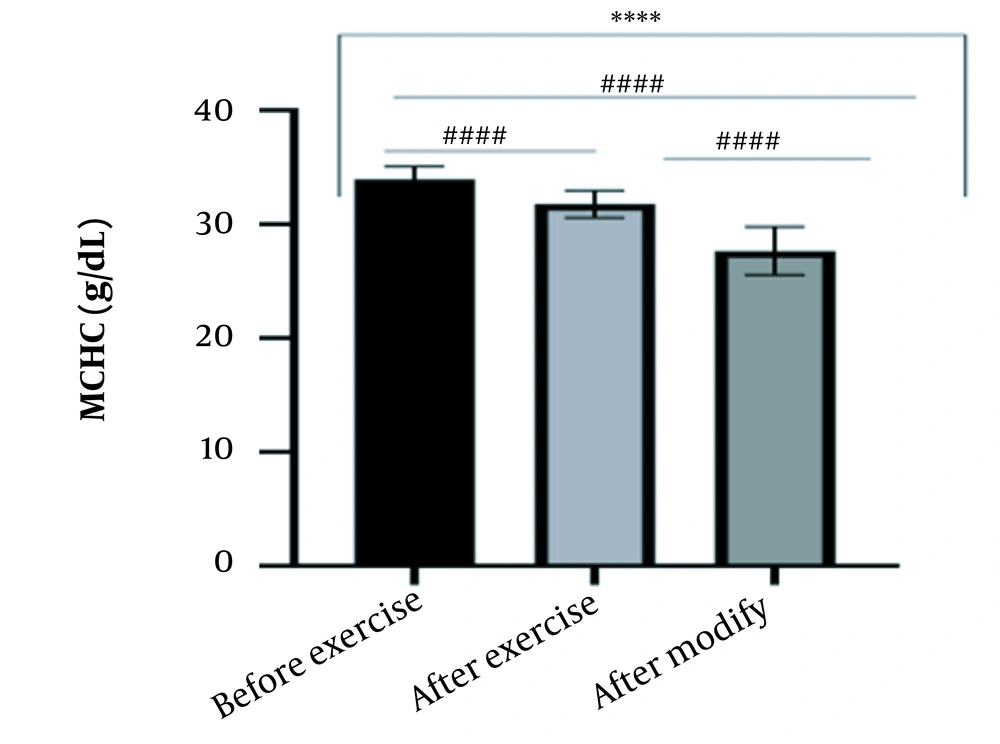
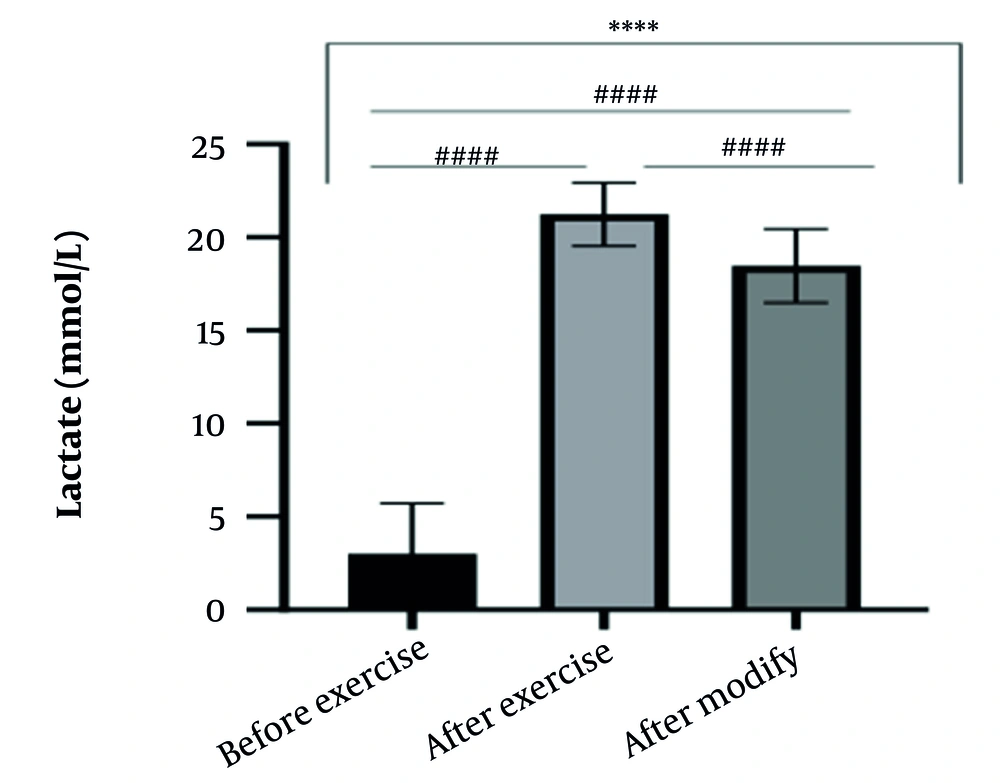
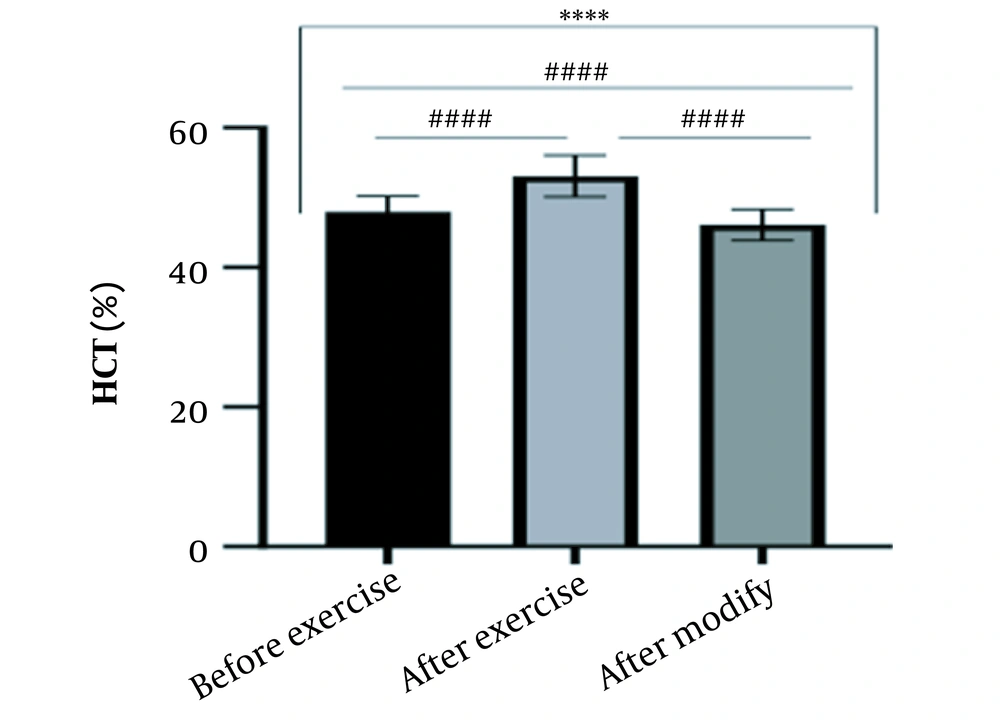
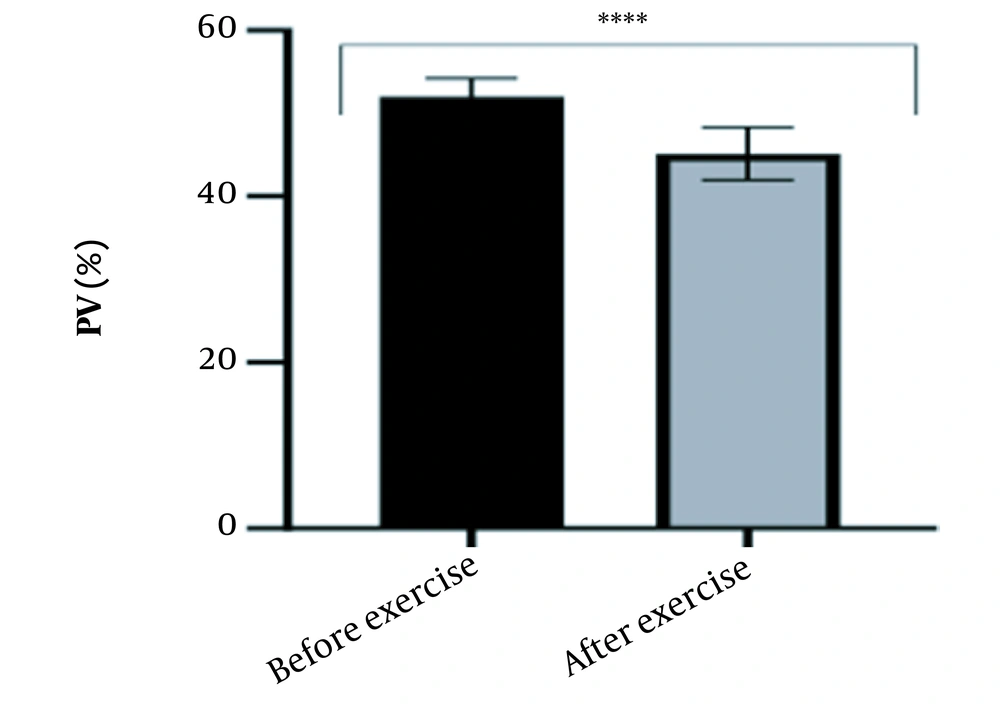
The results of the present study showed that lactate and blood cell levels, including WBCs, Plts, erythrocytes (RBCs), Hb, and Hct increased significantly before modifying the indices. PV decreased significantly (P < 0.0001). The changes in the PV were calculated to be 12.9%. Also, the results of index correction due to changes in PV were such that the rate of WBCs and Plts were still increased significantly (P < 0.0001). While the levels of RBCs, Hb, Hct, MCH, significantly reduced after correction of indices with consideration of PV changes. Table 2 and Figures 1 to 10 have shown the changes in WBC, Plt, RBC, MCV, Hb, MCH, MCHC, lactate, Hct, and PV, respectively.
| Variables | Before Exercise | After Exercise | After Modification | Reference Limit b |
|---|---|---|---|---|
| WBC (X103/μL) | 7.12 ± 1.51 | 13.45 ± 2.03 | 11.70 ± 2.09 | 4 - 11 |
| RBC (106/μL) | 5.78 ± 0.27 | 6.09 ± 0.41 | 5.30 ± 0.39 | 4.5 - 6.5 |
| Plt (103/μL) | 281.83 ± 53.07 | 349.11 ± 73.96 | 303.10 ± 63.85 | 135 - 400 |
| Hb (g/dL) | 16.27 ± 0.9 | 16.83 ± 0.93 | 14.63 ± 0.98 | 13 - 18 |
| Hct (%) | 47.98 ± 2.23 | 53.09 ± 2.96 | 46.10 ± 2.24 | 40 - 54 |
| MCV (fL) | 82.49 ± 3.32 | 87.50 ± 4 | 76.08 ± 4.68 | 76 - 96 |
| MCH (pg) | 27.99 ± 1.30 | 27.79 ± 1.41 | 24.18 ± 1.85 | 27 - 32 |
| MCHC (g/dL) | 33.94 ± 1.16 | 31.77 ± 1.19 | 27.66 ± 2.11 | 30 - 35.5 |
| Lactate (mmol/L) | 3 ± 2.72 | 21.23 ± 1.69 | 18.47 ± 1.98 | 1 - 2 |
| PV (%) | 52.02 ± 2.22 | 45.14 ± 3.18 | - | 46 - 60 |
Abbreviations: WBC, white blood cells; RBC, red blood cells; Plt, platelets; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PV, plasma volume.
a Values are expressed as mean ± SD.
b Reference limits provided are for the general population in rest situations.
4. Discussion
The present study investigated the short-term effects of a single session of CrossFit exercise on some hematologic factors of male athletes. Red blood cells, Hb, Hct, and MCV increased significantly before modifying the indices and without consideration of PV changes and the level of them converted to significant reduction after correction of indices considering PV changes. While MCH and MCHC were reduced after exercise and this reduction became significant after modification. In justifying these changes, we can refer to the effect of PV decreasing due to the high intensity of CrossFit exercise on the initial increase in the above indices. In this regard, it has been shown that a large increase in metabolites such as lactate, ammonium, and potassium in cells reduces the PV through osmosis during exercise (24, 25). Consistent with the results of the present study, Azarbayjani et al. investigated the effect of single session of aerobic and anaerobic exercise on the hematologic profile of young kickboxers and showed a significant reduction of RBC and Hct with consideration of PV changes after anaerobic exercise (26). Minuzzi et al. also assessed hematologic responses to a single session of high-intensity exercise and showed a significant reduction of RBCs, Hb, and Hct 1, 2, 12, and 14 hours after an initial increase in them (27). Belviranli et al. also examined hematologic changes immediately, three and six hours after Wingate exercise that consists of four bouts of high-intensity exercise and four rest bouts between them (5). And showed an increase in RBC, Hct, and Hb. However, these variations were reduced 3 and 6 hours after exercise significantly (5). In justifying the inconsistency between the results of the present study and results of immediately after the high-intensity exercise in Minuzzi et al.’s and Belviranli et al.’s studies, it can be referred to that their results were not modified with consideration of PV changes (5, 27). However, this was done in the present study.in addition, the secondary reduction of RBC counts in the hours after exercise in the above studies confirms that their initial increase was related to a decrease in PV. This issue was also confirmed in our study by consideration of PV changes and showed that the initial increase in RBC, Hb, and Hct indices was related to the decrease in PV. Therefore, the reduction of these indices after consideration of PV changes can be attributed to other mechanisms, such as hemolysis and hematuria of RBCs due to high intensity of CrossFit exercise. In this regard, studies have indicated that the injury caused by abundant and severe muscle contraction or vasoconstriction in internal organs and factors like hypoxia, hyperthermia, dehydration, shear stress owing to hypertension, lactic acid, oxidative stress, proteolysis, and increased concentration of catecholamines can cause or enhance hemolysis due to high-intensity exercise (28). Furthermore, studies have demonstrated that a high increase in lactic acid during strenuous exercise may elevate glomerular permeability; therefore, result in renal excretion of RBCs (hematuria) (29). Therefore, the reduction of RBC, Hb, MCH, and Hct after modification in the present study can be attributed to the very high level of lactate that is increased due to the predominance of anaerobic glycolysis (30). Also, the results of the present study showed a significant increase in the Plts after high-intensity CrossFit exercise. Consistent with this finding, Brahmer et al. showed an increase in Plt, and WBC counts after a single session of progressive exercise to exhaustion on a treadmill (31). Miron et al. also showed an increase in Plt aggregation in young futsal athletes after a single session of high-intensity interval exercise (32). Decrease in PV, increase in the concentration of H in lactic acid, increase in catecholamines and their effect on Plt release from the spleen, and increased mechanical stress such as microvascular injuries due to shear stress due to severe hypertension are the effective factors in the increase in Plt accumulation (33). Considering that in the present study, the Plt count was still significantly high after modifying with consideration of PV changes, it can be said that this increase was independent of PV change and possibly attributed to other mentioned mechanisms. As in the present study, an increase in lactic acid confirms one of these mechanisms. Also, in the case of white blood cells, the results of the present study showed a significant increase, which remained significantly high even with consideration of the changes in PV. This finding is consistent with the findings of Jamurtas et al. and Mathes et al. (21, 34). They showed an increase in WBCs after a single session of high-intensity interval exercise compared to aerobic exercise and resistance exercise, respectively. Also, the findings of Neves et al.’s study showed an increased response of WBCs after strenuous exercise (21, 34, 35). This can be explained by these mechanisms: Hormonal effects, like increased catechol amines, increased micro muscular and microvascular damage that increases the mobilization of WBCs from their storage locations to the site of injury, as well as increased oxidative stress and lactic acid, which are accompanied by the increase in WBCs and inflammatory factors (21, 36, 37). In general, the results of the present study showed that high-intensity CrossFit exercise is an important challenge for the hematologic status of individuals. Because some of these hematologic responses can potentially threaten the health and performance of athletes. Therefore, it is recommended to consider enough time to recover after this exercise.