1. Background
Osteoporosis is a multi-factorial disease associated with the combination of reduced bone mass density and deterioration of the bone structure. Today, osteoporosis is considered as an important health problem for societies and is called the silent disease of the century. This disease is asymptomatic and its complications (fracture) can impose great and non-compensable financial and physical detriments on society and patients (1). Epidemiological studies have shown lower bone density in Asian women than in European and American women. Since women have an 8-fold increased rate of osteoporosis than men, more than half of women aged over 50 years are afflicted with this disease (2).
There are many candidate genes that have been examined for their involvement in the pathogenesis of osteoporosis and bone fractures, including collagen 1a1 (COL1A1), vitamin D receptor (VDR), calcitonin receptor, and osteoprotegerin (OPG) (3, 4). Osteoprotegerin is a soluble member of the tumor necrosis factor receptor superfamily (TNFRSF), which is synthesized by osteoblasts; however, it is also expressed in various organs and tissues, including heart, vessel walls, lung and kidney (5), and is secreted in human milk (6). Osteoprotegerin regulates a variety of cellular processes in immune system (maturation of B cells and development of antibody responses) and bone metabolism (inhibition of osteoclastogenesis) (6, 7). Osteoclastogenesis leads to bone resorption. The balance between bone resorption by osteoclast and bone formation by osteoblast is necessary for bone remodeling process (8). Imbalance of bone remodeling results in different bone diseases, including osteoporosis, osteopenia, osteoarthritis, intervertebral disk degeneration, and idiopathic hyperphosphatasia (Juvenile Paget’s disease) (5, 9-12).
The human OPG is a single copy gene located on chromosome 8, consisting of 5 exons and 4 introns. Expression of OPG gene is upregulated by different regulators, including IL-1β, tumor necrosis factors, TGF-β, vitamin D3, and bone morphogenetic protein 2 (BMP2), and is downregulated by various regulators, such as glucocorticoids and prostaglandin E2 (PG-E2) and parathyroid hormone (PTH) (9, 12, 13). Alterations in promoter and OPG gene sequence could change OPG expression and function (11, 14), and may be associated with osteoporosis and bone mineral density (4, 15). Identifying the molecular mechanisms that regulate OPG and RANKL expression may help to find new targets for bone therapy purposes.
Osteoprotegerin protects bone from excessive resorption through the interaction and inhibition of its ligand, RANKL. Receptor activator of nuclear factor kappa beta is mainly expressed by osteoblastic lineage cells and activated T cells (16, 17). Parathyroid hormone induces RANKL expression (13). Receptor activator of nuclear factor kappa beta is a critical cytokine for the differentiation, activation, and survival of osteoclasts, and a member of the TNF superfamily. To exert its effect on osteoclast, RANKL must interact with its receptor, RANK, located on the membrane of osteoclast. Similar to OPG, RANK is a member of TNFRSF superfamily. The relative concentrations of RANKL and OPG in bone are important determinants of bone mass and strength, and the blockage of RANK/RANKL signaling has become a therapeutic strategy to cure osteoporosis and other disorders related to increased bone resorption (17).
Osteoprotegerin exists either as a 60 kDa monomer or a 120 kDa disulfide-linked homodimer. Each domain of OPG is composed of 7 structural domains. Domains 1 to 4 of the N-terminal site (cystein-rich domain1 (CRD1), cystein-rich domain2 (CRD2), cystein-rich domain3 (CRD3) and cystein-rich domain4 (CRD4)) are cystein-rich domains and have structural similarity to the extracellular CRD domains of TNFRSP proteins, such as RANK. Osteoprotegerin domains CRD2 and CRD3 participate in the interaction with RANKL and prevention of RANKL binding to RANK. It has been also reported that Glu95 (in CRD2 domain) has interactions with RANKL residues Arg223, Tyr241, and Lys257 (18). Exon 2 of OPG contains 3 CRD domains (i.e., CRD1, CRD2, and CRD3). The location of CRD2 and CRD3 domains in exon2 of OPG is displayed in Figure 1.
2. Objective
Due to the importance of CRD2 and CRD3 domains of OPG in the interaction with RANKL, this study aimed to examine exon2 containing CRD2 and part of CRD3 domains in osteoporotic women aged 45 years and older who lived in Chaharmahal va Bakhtiari Province of Iran and referred to Bone Mineral Density Assay Centers in Shahrekord.
3. Methods
3.1. Amino Acid Alignment
To see whether amino acid sequence of CRD2 and CRD3 domains were conserved among mammalian species, the partial amino acid sequence of OPG protein containing exon2 from 3 mammalian species was aligned via Clustal Omega multiple alignment tool (link: www.ebi.ac.uk/Tools/msa/clustalo/).
3.2. Selection of Osteoporotic Women
Out of all women referring to bone densitometry centers of Shahrekord (Chahar Mahal va Bakhtiari Province, Iran), a total of 144 cases were included in this study in years 2014 - 16. First, the participants were asked to complete informed consent forms before entering the study. Patients with a history of corticosteroid use, ovariectomy or early ovarian failure, thyroid disease, impaired calcium absorption, as well as digestive and kidney diseases were excluded from the study. Our study was prospectively reviewed and approved by the ethics committee of Shahrekord Medical Science University (IR.SKUMS.REC.1394.180), and was conducted in accordance with the guidelines and principles of the Helsinki Declaration (19).
Bone density of lumbar spine and femoral neck were determined by dual energy X-ray absorptionmtery (DEXA) using Hologic QDR device (Germany) and were described based on T score method. Then 72 osteoporotic women were selected for further study based on T score.
3.3. DNA Extraction
Genomic DNA was extracted from blood samples using DNA extraction kit (Cinaclone, Iran) according to supplier manual and was stored at -20°C. The quality and quantity of extracted DNA were evaluated using the spectrophotometer (Ultrospec 1100, Amersham Pharmacia Biothech, Australia) and agarose gel electrophoresis (horizontal gel electrophoresis set, paya pajohesh, Iran).
3.4. Polymerase Chain Reaction Amplification
The sequence of exon 2 OPG gene (Gene ID 4982) containing CRD2 and CRD3 domains was amplified using primers designed through Gene Runner v4.0.9.67 Beta and PCR method. The primers used were: Forward, 5’-GTACAGCAAAGTGGAAGA-3’ (as shown by arrowhead in Figure 1) and reverse, 5’-CTCCTAAACTGTCACAACTA-3’ (in intron). As for PCR reaction, 2.5 μL buffer 10X, 0.5 mL MgCl2 50 mM, 0.5 μL Mix 40 mM dNTP, 0.5 μL forward primer 10 pM, 0.5 μL reverse primer 10 pM, 0.2 μL Taq polymerase (0.5 U/μL), and 2.3 μL DNA template (50 ng) were used. The final reaction volume was reached to 25 μL using ddH2O. Thermal condition for PCR reaction was: 95°C for 5 min followed by 35 cycles of 94°C for 30 s, 45°C for 30 s, 72°C for 30 s with final extension at 72°C for 10 min.
3.5. DNA Sequencing
Amplified fragments were further analyzed by adopting DNA sequencing method to show the possible change in exon2 of OPG.
4. Results
The result of amino acid sequence alignment of OPG protein from 3 mammal species using Clustal Omega server showed that human OPG has approximately 81 percent similarity with mouse OPGs, and most of dissimilarities were outside of CRD2 and CRD3 in exon2 (Figure 2). The cystein residues and Glu95 were in the same locations in 3 species.
Partial amino acid sequence alignment of osteoprotegerin from 3 mammalian species. NP_002537.3, NP_032790.3 and NP037002.1 represent amino acid sequence of Homo sapiens, Mus muscullus and Rattus norvegicus, respectively. Black and grey arrowheads show start and end of exon2 and cystein-rich domain2, respectively. White arrowhead shows the start of cystein-rich domain3. Vertical rectangles show Cys107, Glu95, Phe117 Cys118 residues.
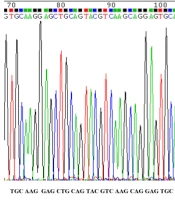
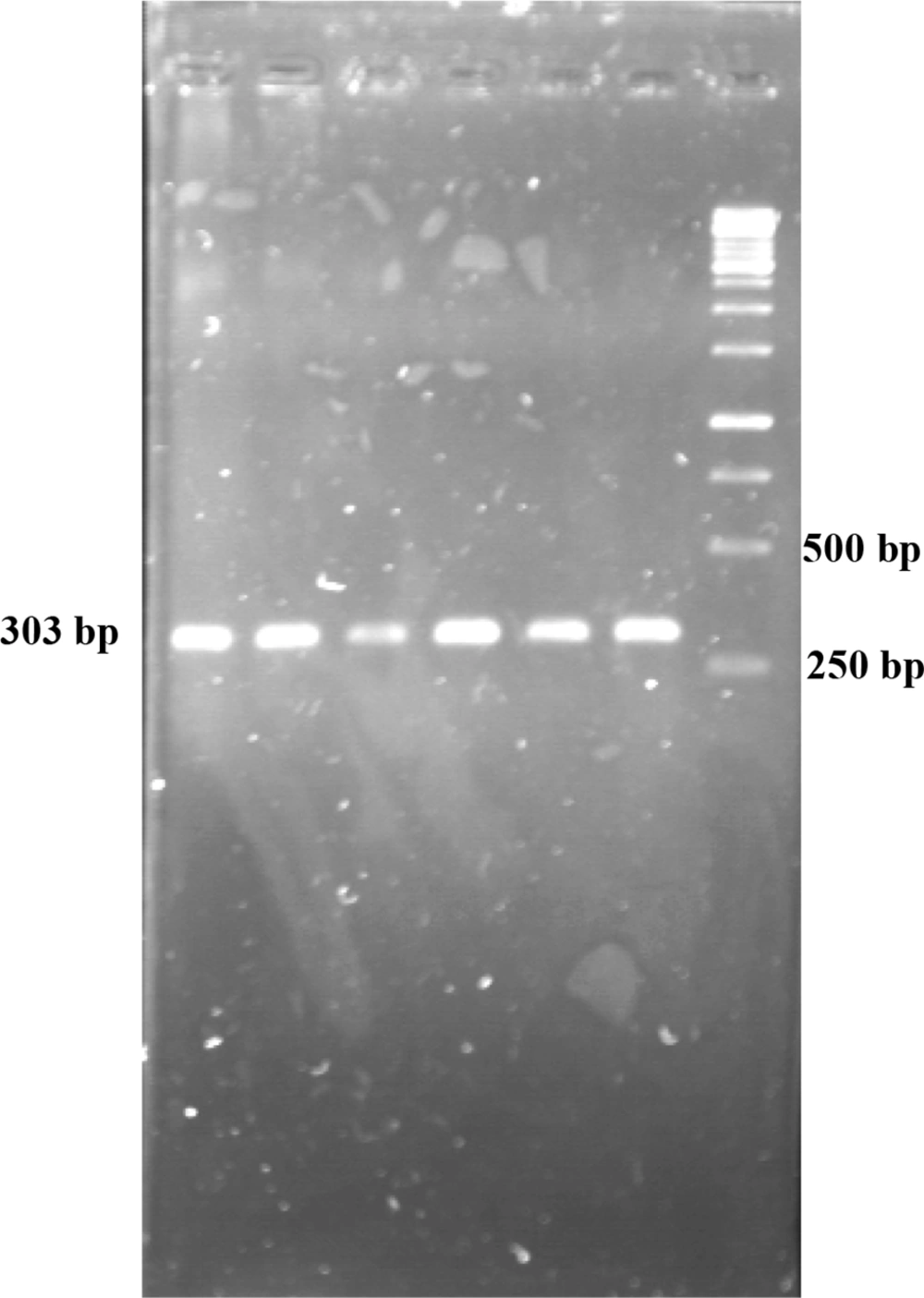
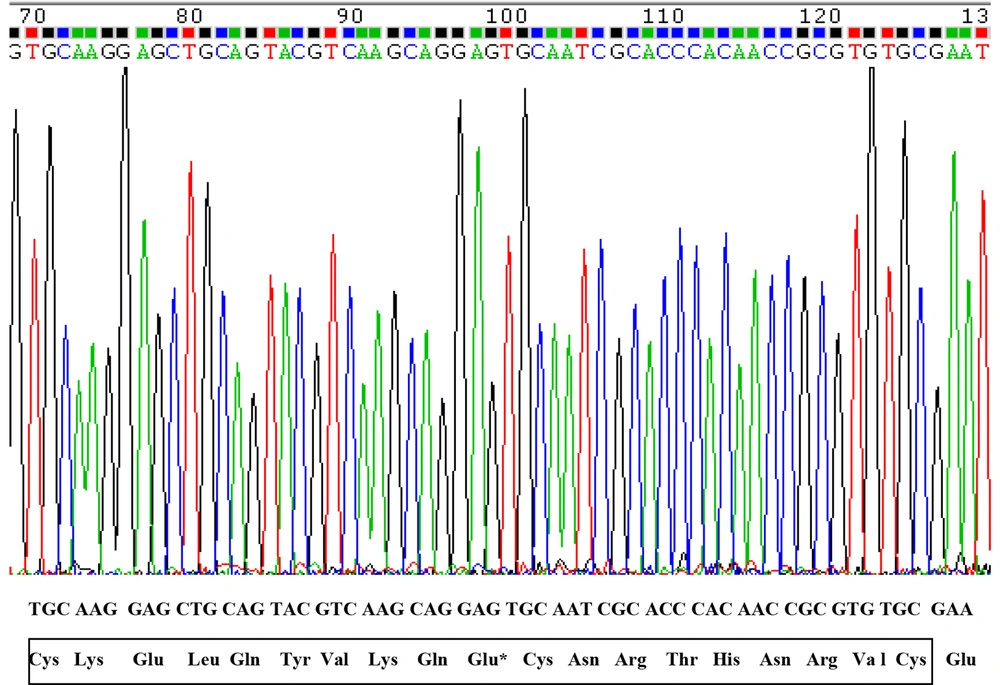
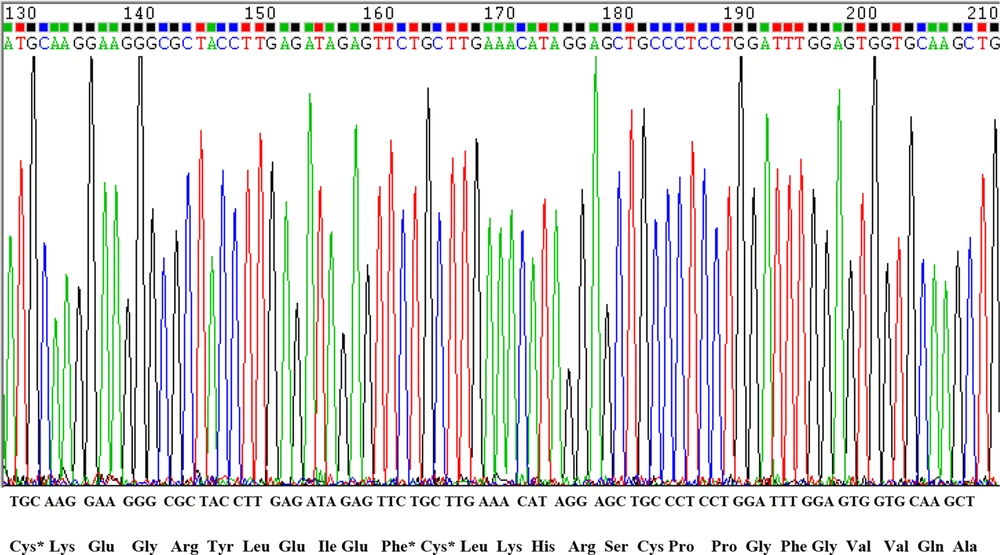
After conducting PCR amplification and gel electrophoresis, the PCR product was observed on agarose gel (Figure 3). The size of product was 303 bp. Polymerase chain reaction products were sequenced and compared with human OPG gene sequence retrieved from NCBI. The results of DNA sequencing of CRD2 and part of CRD3 in exon2 in 72 osteoporotic women did not show any changes in these domains (Figures 4 and 5). As displayed in figures, all cystein residues and Glu95 were intact.
5. Discussion
Osteoporosis is a common and serious disease worldwide. Environmental and genetic factors control the BMD value and, thereby, the osteoporotic fractures. Previous studies have shown that alterations in human OPG gene could be associated with osteoporosis or its phenotypes such as osteoporotic fractures and decrease in BMD (4, 15). Chong et al. suggested the involvement of OPG mutations with the idiopathic hyperphosphatasia (IH) juvenile Paget’s disease phenotype (11). This congenital bone disease is a rare autosomal recessive disorder that causes deformity and physical disability. They identified 6 recessive IH juvenile-associated mutations in OPG gene, three of which were located in exon 2, including Cys65Arg, Cys87Tyr, and Phe117Leu. Alteration of these cysteins to other amino acids destroys disulfide bonds in the second CRD2 of OPG which is the second most effective domain in OPG interaction with RANKL. Furthermore, alteration of Phe117 in CRD3 to other amino acid may affect the formation of disulfide bond between Cys118 and Cys107 (11) and decreases OPG binding to RANKL (20). In this study, therefore, it was decided to examine part of exon2 of OPG containing CRD2 and part of CRD3 in order for identifying possible mutation in 72 osteoporotic women in Chahar Mahal va Bakhtiari Province of Iran. It was found that majority of amino acids -cysteins, in particular- were conserved in CRD2 and CRD3 in comparison with mice OPG, and that these regions were intact in osteoporotic women. This may have implied that there was no defect in the interaction between OPG and RANKL in these osteoporotic women. Moreover, it was indicated that women who were homozygous for Phe117 had higher spine and neck hip BMD in comparison with those who were heterozygous (Phe/Leu) or homozygous (Leu/Leu) for position 117 (21). The same situation was found for women who were homozygous for Cys87 in comparison with those who were heterozygous (Cys/Tyr) or homozygous (Tyr/Tyr) for position 87 (22).
Since osteoporosis is a multifactorial disease, there might be another reason for having low BMD and frequent bone fracture in present cases. It has been argued that the ratio of RANKL to OPG is important for the regulation of osteoclast differentiation and function (8). It has been also indicated that the level of OPG in patients with a long bone fracture is higher than that in healthy controls. In contrast, the level of RANKL decreases during regular fracture repair (23). Alterations in promoter of OPG gene could change OPG expression (15) and may be associated with osteoporosis. Alteration in four locations in OPG promoter, -960, -946, -900, and -864, either alone or in combination, produces different haplotypes and affects the ability of transcription factors (e.g., specificity protein1 (Sp1) and NF-1) to attach to the region containing these sites, leading to various levels of transcription (14).
Moreover, the degree of methylation of CpG sites in the CpG island of OPG/RANKL promoters affects these genes expression, so that higher methylation degree causes lower gene expression (9).
In addition to OPG, an association was detected between some single nucleotide polymorphisms (SNPs) in COL1A1, VDR, and calcitonin receptor genes with bone density in Iranian osteoporotic women living in Chahar Mahal va Bakhtiari Province, Iran (24-26). Taq1 and Bsm1 polymorphisms of VDR as well as Sp1 polymorphism of COL1A1 were also found to have a significant association with bone density (P < 0.05) (18, 19). However, it was recommended that further studies should be carried out to examine OPG expression in these women and extra cases and show the role of OPG in order for finding more information about the pathogenesis of osteoporosis in osteoporotic women.
5.1. Conclusions
Since the majority of amino acids and specially cysteins were intact in the studied osteoporotic women, it was concluded that there was no defect in interaction between OPG and RANKL. Due to the importance of OPG protein in bone remodeling, however, it was recommended OPG gene expression in osteoporotic women should be further investigated.
5.2. Limitations and Suggestions
Due to time and financial limitations, performing extra PCR amplification and DNA sequencing was not possible. Therefore, it was suggested that a larger sample of osteoporotic women should be selected and investigated in order for identifying possible mutation in CRD2 and CRD3 domains.