1. Background
Invasive procedures such as bone marrow aspiration and biopsy are now an integral part of diagnostic and therapeutic procedures for children with cancer.
These procedures are painful and often more difficult to bear than the disease itself and cause great anxiety in this group of children and their parents. For this reason, sedation and analgesia used for these diagnostic and therapeutic procedures are increasingly expanding in pediatric hematology-oncology departments (1).
For this purpose, in addition to psychological support programs, drugs are used for sedation and analgesia in these children (2). Due to the short duration of these measures, it is necessary to use drugs that effectively relieve patients’ anxiety and pain and act shortly, with significant drowsiness or side effects for the patient after the procedure (3). Today, various drugs such as propofol, ketamine, fentanyl, alfentanil, remifentanil, midazolam, or a combination of them are used for analgesia and sedation in children. The choice of these drugs depends on the procedure location (ward or operating room), procedure type (bone marrow biopsy, aspiration, or lumbar puncture), child’s age, and whether the patient is outpatient or inpatient.
Midazolam is a benzodiazepine drug with anti-anxiety and sedative properties commonly used in both children and adults for various diagnostic and therapeutic procedures due to its short duration and rapid onset of action. This drug prevents unpleasant memory of the painful procedure with antegrade amnesia. However, due to the lack of analgesic properties, this drug should be used during painful procedures in combination with an analgesic drug (4, 5).
Ketamine is a derivative of phencyclidine. This drug has sedative and analgesic properties that can be used alone or in combination with other drugs to cause analgesia during diagnostic and therapeutic measures in children. This drug can be administered orally, intravenously, intramuscularly, and intrathecally (6, 7). Ketamine has a shorter half-life in children than adults (8, 9). Therefore, the applied dose and its side effects would differ between children and adults. The side effects mainly include salivation, increased bronchial secretions, drowsiness, nausea/vomiting, restlessness, visual hallucinations or nightmares, seizures, flushing, and laryngospasm or bronchospasm (10). These side effects are dose-dependent and should be avoided in children (11). A single dose of midazolam in the range of 0.05 - 0.1 mg/kg in short-term use will usually have a short recovery time, which varies from person to person. Ketamine in combination with benzodiazepines is usually required at lower doses. Ketamine in 0.25 - 0.5 mg/kg can provide sedation and analgesia in painful procedures, but sufficient and complete sedation in the range of 1 to 2 mg/kg can be developed slowly until general anesthesia (12).
2. Objectives
This study aimed to evaluate the effectiveness of intravenous midazolam and ketamine combination in bone marrow aspiration and biopsy in children with cancer in the pediatric hematology-oncology ward.
3. Methods
This descriptive cross-sectional study was done on 100 children aged six months to 17 years admitted to the pediatric hematology-oncology ward of Ali-ebne Abitaleb hospital who were candidates for bone marrow aspiration or biopsy due to cancer. Sampling was easy, accessible, and sequential.
Inclusion criteria included age of six months to 17 years, a malignancy requiring bone marrow aspiration or biopsy, fasting for at least six hours prior to drug injection, and American Society of Anesthesiologists (ASA) class 1 to 3. Exclusion criteria included patient dissatisfaction, hemodynamic instability, history of respiratory diseases and asthma, history of allergic reactions to one of the drugs used, the presence of brain tumor, decreased level of consciousness, seizures, and ASA class 4 or more. We obtained the approval of the ethics committee and written consent of the parents of patients admitted to the pediatric hematology-oncology ward of Ali-ebne Abitaleb hospital, who were candidates for bone marrow aspiration or biopsy due to cancer.
First, a safe venous route was implanted with 22-gauge angiocatheters, and then the patient was transferred to an outpatient operating room for complete monitoring, including pulse oximetry, heart rate, respiration rate, blood pressure, and electrocardiography. After confirming the patient's condition, an anesthesia assistant injected midazolam 0.05 mg/kg and ketamine 1 mg/kg. Then, an oxygen mask at 4 to 6 L/min was established for the patients. One minute after injecting the drug combination, the patient's sedation level was calculated based on the modified Ramsey score. However, if the patient needed more sedation agents, the supplement dose of midazolam would be injected at 0.05 mg/kg and ketamine at 0.4 mg/kg. In such a case, the patient was excluded from the study. If arterial saturation decreased to less than 90% of patients, it was ventilated by T-piece or Ambu-bag. Resuscitation drugs, including atropine, adrenaline, lidocaine, and succinylcholine, along with a laryngoscope set and endotracheal tubes, were available in the outpatient operating room emergency trolley. Anesthesia medication was injected, and sedation was determined based on the modified Ramsey score by the anesthesia assistant. An experienced and trained nurse recorded the complications.
We used the mean and standard deviation for quantitative data such as age and sedation degree and frequency and percentage for qualitative variables.
4. Results
In this study, 100 children aged six months to 17 years who were candidates for bone marrow aspiration or biopsy admitted to the pediatric hematology-oncology ward of Ali-ebne Abitaleb hospital were studied, with a mean age of 6.8 ± 4.3 years (Table 1).
| Age (y) | No. (%) |
|---|---|
| < 2 | 11 (11) |
| 2 - 5 | 35 (35) |
| 6 - 10 | 29 (29) |
| 11 - 17 | 25 (25) |
| Total | 100 (100) |
Frequency of Patients in Age Groups
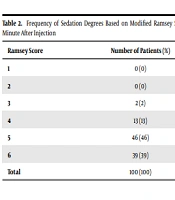
The mean sedation degree in pediatric patients was 5.2 ± 0.74 based on the modified Ramsey score one minute after midazolam and ketamine injection, and the highest frequency was observed in Ramsey score 5 (Table 2).
| Ramsey Score | Number of Patients (%) |
|---|---|
| 1 | 0 (0) |
| 2 | 0 (0) |
| 3 | 2 (2) |
| 4 | 13 (13) |
| 5 | 46 (46) |
| 6 | 39 (39) |
| Total | 100 (100) |
Frequency of Sedation Degrees Based on Modified Ramsey Scores One Minute After Injection
In this study, nine (9%) patients developed nausea, but no case of vomiting was observed. Also, six (6%) children experienced arterial saturation less than 90% after midazolam and ketamine injection, which was resolved by an oxygen mask. However, none of the patients developed apnea and did not need to be ventilated with Ambu-bag. Twelve (12%) children developed restlessness on waking up after injecting midazolam and ketamine. Besides, none of the children in this study developed laryngospasm or bronchospasm.
5. Discussion
The present study showed that the mean degree of sedation based on the modified Ramsey score was 5.2, which is acceptable. Nausea and vomiting were seen in 9%, decreased arterial saturation in 6%, and restlessness in 12% of children. There were also no cases of laryngeal spasms in children.
Some studies in this area, including the study by Cheuk et al., examined the effectiveness of midazolam and ketamine in children undergoing outpatient surgery. The results showed that these drugs provided rapid, effective, and safe sedation, and some side effects, such as reduced arterial saturation, occurred dose-dependently. In this study, after the first 30 seconds, 75% of the patients needed only one dose of 0.1 mg/kg midazolam with 1 mg/kg of ketamine, which is consistent with our study (10). Another study used a combination of benzodiazepines and ketamine for sedation before a renal biopsy. Out of 60 children studied, only six children experienced mild arterial oxygen saturation reduction, and none of them had a significant change in respiration rate or blood pressure. Sedation quality was optimal in 53 patients and acceptable in seven patients. None of the patients required intubation or ventilation (13).
In a study by Novak et al., sedation with ketamine and midazolam was evaluated at a dose of 0.05 mg/kg. The results showed that these drugs were beneficial at low doses and with careful monitoring. Among significant complications in a small number of patients was laryngospasm, while our study did not show any cases of laryngospasm. However, similar to our study, a few side effects such as nausea, vomiting, hallucinations, and dizziness were observed (14). In addition, a study by Darabi et al. compared the efficacy of midazolam-ketamine and propofol-alfentanil in children undergoing bone marrow aspiration. The results showed the effectiveness of both drug combinations. The difference was that a decrease in systolic blood pressure and heart rate was observed in the propofol-alfentanil group, unlike the midazolam-ketamine group, which showed increases in both cases (15). The efficacy of midazolam-ketamine was assessed compared with midazolam alone in another study. There were 40 children in this study, and similar to our study, most of the children were under seven years old. The results showed that the differences between midazolam sedation and midazolam-ketamine combination were statistically significant. Sedation was greater in older children with midazolam and ketamine combination. As a result, the midazolam-ketamine combination was more successful for sedation and pain relief than midazolam alone (16).
A study by Dili et al., comparing ketamine alone and midazolam-ketamine combination in 99 children aged two to 14 years with a mean age of 6.5 years, found that the sedation time was significantly shorter in the midazolam-ketamine group. Parental satisfaction was higher in the midazolam-ketamine group. There was no significant difference in recovery time and nausea/vomiting between the two groups, but the child's restlessness (nightmares and cries) was more in the ketamine group (17). A study by Acworth et al. assessed the rate of intranasal midazolam sedation compared with intravenous midazolam-ketamine in children requiring emergency sedation. The results showed that the midazolam-ketamine combination was an excellent way to achieve adequate sedation for most painful procedures in emergency department children (18).
In conclusion, the present study showed that the midazolam and ketamine combination provide suitable sedation to children, and more importantly, its side effects are minimal and negligible. According to the results of our study and other studies, it is proposed to use sedatives such as ketamine and midazolam in children undergoing invasive and painful procedures such as lumbar puncture, bone marrow aspiration, and or biopsy.