1. Background
As the most prevalent noncontagious neurologic condition, epilepsy is an important cause of mortality and disability in children. Epilepsy is defined as an “enduring” tendency to seizures, but “enduring” is not easily defined (1). In addition, based on practical reasons, epilepsy is defined as ≥ 2 seizures unprovoked and separated by at least a period of 24 hours. This is based on the concept that the risk of recurrence after 2 unprovoked seizures is about 80%, while the risk of recurrence after a single unprovoked seizure is only about 50% of cases (2-4). The epilepsy concept of 2 unprovoked seizures is useful in patients with dramatic convulsive seizures. The newborn period is an important interval for epilepsy; also, many of them have seizures due to acute encephalopathy (5, 6).
Some related risk factors are as follows:
(1) Age, the onset of epilepsy is common in children and older adults, but the condition can occur at any age;
(2) Family history;
(3) Head injuries;
(4) Stroke and other vascular diseases;
(5) Dementia;
(6) Brain infections;
2. Objectives
There is a clear bias in referred patients or cases referred to the emergency department, especially in countries without prepaid comprehensive health care (10). A successful method has been to search requests for electroencephalogram (EEG) recordings, provided that they serve a regional population (11-13). These show the importance of this condition; thus, this study aimed to evaluate and investigate the causes and risk factors of epilepsy and seizures in children.
3. Methods
3.1. Study Population and Setting
The inclusion criterion was children with seizures admitted to the Pediatric Clinic of Amir-Kabir Hospital from April to September 2008. Exclusion criteria were all patients who were not willing to participate in the study and those who had not required EEG; the indications of EEG in epilepsy included recurrent cases, non-response to treatment, abnormal cases, etc. Also, patients who had been discharged before the test results were excluded from the study. Based on our criteria, we enrolled 291 patients with seizures aged 2 months to 12 years.
3.2. Measurements
First, accurate biography of the parents was taken to rule out other differential diagnoses of seizures. After the diagnosis of seizures, age, gestational age, birth weight, and type of delivery were recorded. Also, we took a detailed history of seizures in patients and parents, type of seizure, the duration, and frequency of seizure, febrile seizure, presence of disease, conditions susceptible to seizure, head trauma, drug uses in 7 days before seizures, and discontinuation of anticonvulsant drugs. In addition, a neurologic examination was performed, and the process of growth and development of children was recorded. Then, we took EEG, magnetic resonance imaging, and computed tomography scans in children with indications for these tests.
3.3. Ethical Considerations
In this study, the names and details of the individuals were kept confidential, and no costs were imposed on the patient’s family and the hospital. All information on the history and clinical examination, EEG, brain imaging, and tests was obtained with the full consent of the children’s parents. Informed consent was obtained from all children’s parents prior to their participation in this study. Researchers were required to comply with the provisions of the Helsinki Declaration at all stages of the research process, including writing a proposal, collecting samples, and analyzing them. Also, the protocol of the study was approved by the Ethics Committee of Arak University of Medical Sciences (code: IR.ARAKMU.REC 4-35-87).
3.4. Statistical Analysis
Data were analyzed using SPSS version 24 (SPSS Inc, Chicago, Ill, USA); we used mean and SD for descriptive information.
4. Results
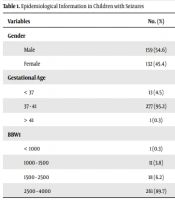
Epidemiological study evaluation showed that 54.6% of children with seizures were male, 95.2% were in 37 - 41 weeks, 4.5% were lower than 37 weeks, and 0.3% had a gestational age of more than 41 weeks. In addition, the most common body birth weight of children was 2500 to 4000 g in 89.7% of children with seizures (Table 1).
| Variables | No. (%) |
|---|---|
| Gender | |
| Male | 159 (54.6) |
| Female | 132 (45.4) |
| Gestational Age | |
| < 37 | 13 (4.5) |
| 37 - 41 | 277 (95.2) |
| > 41 | 1 (0.3) |
| BBW1 | |
| < 1000 | 1 (0.3) |
| 1000 - 1500 | 11 (3.8) |
| 1500 - 2500 | 18 (6.2) |
| 2500 - 4000 | 261 (89.7) |
Abbreviation: BBW, body birth weight (g).
The generalized tonic-clonic seizure was the most prevalent seizure observed in 134 cases (46%), 281 cases (96.6%) had no history of previous seizures, and 277 cases had no history of seizures in their brothers or sisters (Table 2). A review of drug history in children showed that 190 cases (65.4%) had no drug consumption, and infant acetaminophen drops were the most common drug in these patients (Table 3). Further, 141 cases (48.6%) had no history of related conditions, and upper respiratory tract infection was the most common condition (Table 4).
| Variables | No. (%) |
|---|---|
| Types of seizures | |
| Simple partial | 1 (0.3) |
| Complex partial | 8 (2.7) |
| Clonic | 3 (1) |
| Generalized tonic-clonic | 134 (46) |
| Tonic | 92 (31.6) |
| Atonic | 40 (13.7) |
| Myoclonic | 13 (4.4) |
| Seizure history | |
| Without seizure | 281 (96.6) |
| Seizure without fever | 1 (0.3) |
| Seizure with fever | 6 (2.1) |
| Epilepsy | 3 (1) |
| Seizure in brother and sister | |
| Without seizure | 277 (95.2) |
| Seizure without fever | 1 (0.3) |
| Seizure with fever | 11 (3.8) |
| Epilepsy | 2 (0.7) |
| Seizure duration (min) | |
| < 15 | 266 (91.4) |
| 15 - 30 | 22 (7.6) |
| > 30 | 3 (1) |
| Fever with seizure | |
| Non | 90 (26.1) |
| Before seizure | 191 (55.5) |
| During seizure | 4 (1.2) |
| 2 days after seizure | 6 (1.7) |
| Drugs | No. (%) |
|---|---|
| None | 190 (65.4) |
| Acetaminophen drops | 28 (9.6) |
| Ibuprofen syrup | 9 (3.1) |
| Acetaminophen suppositories | 2 (0.7) |
| Amoxicillin syrup | 6 (2.1) |
| Erythromycin syrup | 3 (1) |
| Cephalexin syrup and acetaminophen drops | 1 (0.3) |
| Amoxicillin syrup and acetaminophen drops | 5 (1.7) |
| Penicillin injections | 2 (0.7) |
| Cold syrup of children | 4 (1.4) |
| Guaifenesin syrup | 1 (0.3) |
| Sodium valproate syrup | 6 (2.1) |
| Carbamazepine syrup | 2 (0.7) |
| Phenobarbital tablets | 9 (3.1) |
| Sodium valproate syrup and phenytoin | 5 (1.7) |
| Phenobarbital and carbamazepine | 5 (1.7) |
| Sodium valproate syrup and phenobarbital and carbamazepine | 1 (0.3) |
| Metoclopramide | 1 (0.3) |
| Other medications | 3 (1) |
| Diseases | No. (%) |
|---|---|
| Non | 141 (48.6) |
| Pneumonia | 10 (3.4) |
| Bronchiolitis | 14 (4.8) |
| Gastroenteritis | 33 (11.3) |
| Pharyngitis | 6 (2.1) |
| Vaccine | 4 (1.4) |
| Upper respiratory tract infection | 76 (26.1) |
| Roseola infantum | 2 (0.7) |
| Infectious mononucleosis | 1 (0.3) |
| Croup | 1 (0.3) |
| Otitis media | 1 (0.3) |
| Other diseases | 2 (0.7) |
5. Discussion
In this study, we did not get any good results on the effects of head trauma, withdrawal of antiepileptic drugs, and results of EEG and brain imaging. This is because of the low frequency of cases. However, based on our results, we could find some ways to better control seizures and epilepsy by considering risk factors, including infective disease such as gastroenteritis and pneumonia.
In the following paragraphs, other studies were evaluated. In the study by McNelis et al., age was an unrelated factor in recurrent epilepsy after febrile convulsion (14). This is not consistent with the results of Saliba et al., who studied the risk factors for neonatal seizures, which had a higher prevalence of seizures in preterm infants weighing 1500 g and in term infants with small for gestational age (SGA) (15). This difference may be due to the different age groups of children evaluated in the 2 studies. In addition, one of the most important risk factors of neonatal seizures in term neonates is cesarean section delivery in the study of Saliba et al. (15). This discrepancy may be due to indications for cesarean section. Some of them may cause hypoxia and brain injury and eventually lead to symptomatic seizures in the neonate, and symptomatic seizures can lead to neonatal death due to underlying diseases (15). However, our study was performed on children after 2 months of age; thus, we could not evaluate this index in the cases.
In the present study, among children with a previous history of seizure, the most prevalent risk factor was a history of febrile convulsion. This finding is consistent with Mung’ala-Odera et al. and Matuja et al. studies, finding that a seizure history was a risk factor for epilepsy (16, 17). In addition, in the study of seizure history in the second- and third-degree relatives of 291 studied children, most of them had no history of seizures. This finding is consistent with the study by Tsai and Hung, considering family history of febrile seizure as an unrelated factor in recurrent epilepsy after seizure (18). However, Daoud et al., Asadi-Pooya and Hojabri., and Masri et al. showed a family history as a risk factor for epilepsy (19-21). The reason for this discrepancy is the descriptive nature of our study. The generalized seizure was the most prevalent seizure among the studied children. This finding is in line with the study of Verity et al., who described the most common form of seizure as tonic-clonic and partial complex (22). Most of the studies conducted on these seizure risk factors indicated the importance of evaluating these risk factors to better control seizure and epilepsy.
5.1. Conclusions
Infectious diseases (such as gastroenteritis and pneumonia) were observed as the most important possible risk factors in this study. Thus, based on our results, we could find some ways to better control seizures and epilepsy by considering risk factors, including infective disease such as gastroenteritis and pneumonia.