1. Background
The liver, as the largest internal organ in the body, weighs about 1.5 kg on average in adults. Hepatocytes are the main cells of this organ and the most active cells in the body. In addition to their exocrine function by bile production, these cells, along with other liver cells, process the contents of the blood by functions such as synthesis and endocrine secretion of major plasma proteins, gluconeogenesis, detoxification, deamination, and the storage of glucose, triglyceride, vitamin A, and iron. Liver cells normally have a low rate but a high capacity for regeneration. Surgical removal of some parts of the liver or destruction of hepatocytes due to toxins stimulates mitosis in healthy hepatocytes, and by the process of compensatory hyperplasia, the original tissue mass is preserved (1, 2). Acute liver failure (ALF) occurs when the rate of hepatocyte death exceeds the liver’s capacity to regenerate. Acute liver failure is defined as the development of hepatic encephalopathy and coagulopathy, which occurs within 26 weeks of severe liver injury in a patient with no previous history of liver disease. Even with current medical care, ALF can progress rapidly to coma and death. The most common causes of death are increased intracranial pressure and cerebral edema, followed by failure of a number of other body systems. The most common causes of ALF are viral hepatitis, autoimmune hepatitis, drugs-toxins-induced hepatitis (such as herbal drugs), and some pregnancy-related conditions. In one study conducted by Molinari et al. on green tea extract using a pill-like supplement for weight loss, the toxicity of this extract on specific hepatocytes was confirmed by blood tests and histopathology examination (3). Another study by Goldberg et al. showed that drugs were one of the unusual causes of ALF, but over-the-counter medications and herbal supplements were the most common causes (4). Amaranthus caudatus (amaranth) is an ancient American crop that dates back to BC. It now grows in Mexico, Peru, India, and West Africa, especially in mountainous areas (2). The red color of the amaranth plant is used every year in foods (such as soft drinks), sweet powder, candy, medicines (pill covers), and cosmetics (lipsticks) in more than 60 countries (5).
Some studies on amaranth have shown cholesterol-lowering effects. In an experiment performed on rabbits, they observed a 50% reduction in triglycerides as well as a reduction in cholesterol when fed with this plant (6, 7). Amaranth leaves are an excellent source of protein, fiber, squalene, anthocyanins, and tocotrienol. Squalene is an intermediate component in cholesterol biosynthesis and has been shown to have antioxidant and anti-inflammatory effects in vitro. The amaranth plant contains tocotrienol and tocopherol, which regulate cholesterol levels (8).
In one study conducted by Zeashan et al. in 2009, they examined the hepatoprotective and antioxidant effects of this plant. They revealed it had a significant hepatoprotective activity that might be due to antioxidant factors and phenolic compounds (9). Yawadio Nsimba et al. examined the antioxidant capacity of amaranth seed extract, which confirmed the antioxidant properties of this plant (10). Another study by Al-Dosari on ethanolic extracts of amaranth leaves against hepatotoxicity induced by carbon tetrachloride (CCl4) in rats showed that oral administration of this substance for three weeks reduced serum levels of liver enzymes (AST, ALT, and GGT), bilirubin, and cholesterol. Acute toxicity test showed that this extract did not cause any side effects or mortality at the administered dose, and the hepatoprotective effect was due to its antioxidant properties (11). Zeng et al. examined the effects of antioxidant therapies on enhancing recovery in patients with acute liver failure (12).
In another study by Collins and McLaughlin, they injected red dye amaranth extract into the gastric tube of female rats at different doses and then examined the delivery and health of the embryos, which showed that a high number of deaths in delivery occurred at high doses. This indicated the systemic effects and fetotoxicity of the compound (5). In another study performed on rats by Ashok Kumar et al., the rats were divided into five groups and were given sodium carboxymethyl cellulose plus 200 and 400 mg/kg of methanol extract from red amaranth for 14 days later. The results showed that consumption of 200, 400 mg/kg of red amaranth extract had a hepatoprotective role and resistance to paracetamol (acetaminophen). Histopathological examination of the liver confirmed the increased hepatoprotective properties of this substance (13). A study by Baig on the hepatic effects of red amaranth on mice showed an increase in liver enzymes, serum bilirubin, decreased serum protein and albumin, sodium, and potassium concentrations, and prolonged clotting time, which well proved the hepatotoxic effects of the plant (14).
In the past few years in the gastrointestinal ward of Afzalipoor Hospital in Kerman, anecdotal reports showed in some cases, consumption of unsuitable extracts of amaranth could cause ALF. It was questioned whether amaranth consumption caused liver failure, so we decided to study its effects on growing human hepatocytes via in vitro interactions.
2. Objectives
The present study was performed to determine the cytotoxic and lethal effects of amaranth on human hepatocyte cell line and evaluate the viability of hepatocytes after the effect of the amaranth drug. We also investigated the appropriate dose and IC50 of the amaranth drug on hepatocytes for future animal studies.
3. Methods
3.1. Amaranth Plant Extraction
This research was an experimental/interventional study, and the extract of the amaranth plant was prepared and standardized in Kerman School of Traditional Medicine during laboratory stages. For the first extraction, the plant was ground into a coarse powder using an electric grinder and passed through a 20 mesh sieve. Then each 100 g of plant powder was soaked in 600 cc of 50% alcohol for 24 hours in a dark environment at room temperature (RT). The resulting extract was then filtered using a Buchner funnel, and the solvent was added again to the remaining pulp. This was repeated four times to remove all the compounds in the plant. Finally, the collected extracts were added to each other and concentrated at 50°C in a rotary distillation apparatus under vacuum and dried in an oven at 50°C. For later use, the plant extracts were stored in a freezer at -20°C.
3.2. Preparation of Hepatocytes
Hepatocytes were obtained from the cell bank of the hydatid cyst research center of Afzalipour Medical School. First, the vials containing hepatocyte cells were removed from the nitrogen tank and incubated for a few minutes at room temperature, then placed in a pan with a temperature set at 37°C to melt the contents of the vial. Immediately the contents were transferred to a 1.5 cc microtubule and centrifuged at 1,500 revolutions per minute (RPM) for three minutes. Then, we discarded the contents of the microtube and homogenized the cells adhering to the bottom of the dish by adding culture medium. Then, some of the new medium containing the cell was poured into a 5 cc plate, and the culture medium was added to a volume of 5 cc. These cells were placed in five Dulbecco’s modified eagle medium (DMEM) media with 10% fetal bovine serum (FBS) and penicillin, and streptomycin and incubated at 37°C in 95% humidity and 5% CO2. The culture environment was changed three days later and then every two days. To replace the culture medium, the contents of the flask were emptied, the bottom of the flask was washed with 3 mL PBS, and 5 mL of new 10% FBS medium was added to the flask.
After growth and proliferation, the cells were stored and frozen, fresh cells were used for cell tests, and frozen cells were transferred from the incubator to the bottom of the hood to return to normal (when the cell plate layer of the plate was about 80). Approximately 1.5 cc of trypsin was added to each plate and incubated for three minutes. Then, we transferred the cells floating inside the trypsin to a 1.5 cc microtube and centrifuged at 3,500 RPM for three minutes. The trypsin content was then discarded from the microtube, and the cells adhering to the bottom of the dish were homogenized in a culture medium and finally cultured at lower cell densities. After a few weeks of passage, we started treating the cells for cell survival tests. After counting the cells with a neobar slide, 5,000 of them were poured into a 96-well plate. Then, 200 μL complete DMEM medium was added to each well. The plate containing the cells was kept in an incubator for 24 hours to let the cells adhere to the bottom. After 24 hours of incubation, the wells were completely evacuated.
3.3. Treatment of Cells
Hepatocytes were placed in four media containing amaranth extract and treated with dilutions of 10, 50, 100, and 200 μg/mL and a control culture medium (complete new DMEM medium). In each culture medium, the dilution of amaranth extract was measured accurately by laboratory methods. After 24 hours of incubation, using cell survival tests, including MTT assay and neutral red, the percentage of cell growth and proliferation in each dilution of the amaranth plant was measured and compared with the control group.
3.4. Cell Viability and Cytotoxicity Assays
3.4.1. MTT Assay
MTT is a yellow compound that, after entering the mitochondria of living cells, is regenerated by the enzyme reductase and converted to a purple substance called formazan. Therefore, in cases where there are more living cells, due to the higher activity of mitochondrial reductase enzyme, MTT is converted to more formazan, and as a result, more purple color is produced. To perform this test, after 24 hours of incubation in different treated environments, 0.05 mg of MTT was added to each well and kept in the incubator for two hours. Subsequently, all the liquid in the wells was drained, and 100 microliters of dimethyl sulfoxide (DMSO) was added to each well (DMSO by enhancing cell membrane permeability and dissolving the formazan causes its purple color to be spread around the wells). The plate was gently shaken several times to fully disperse the purple dye in a good environment. In the end, the absorbance, or optical density (OD) of the purple dye of the wells, was read by an ELISA reader at a wavelength of 490 nm. All steps were repeated five times, and the mean absorbance was considered the mitochondrial activity. The percentage of cell growth and proliferation in each dilution was determined by the following equation:
Percentage of living cells = [The intensity of absorption in the presence of drug - Blank absorption] / [Intensity of control group absorption - Blank absorption intensity] ×10
3.4.2. Neutral Red Assay
Neutral red is a weak cationic dye that diffuses easily through the membrane of lysosomes and accumulates in the lysosomes by binding to anionic sites in the lysosome matrix. In damaged or dead cells, the cell membrane does not exist or acts as a barrier to prevent dye from leaving the cells, so where more dye has accumulated, it indicates the presence of more living cells. To perform this test, the contents of the wells were drained after 24 hours of incubation in different treated environments, 200 μL of the desorbing solution was added to each well, and neutral red dye was removed from the cells and accumulated in the wells. Then a 96-well plate was placed on a shaker for about ten minutes. Eventually, the absorbance of the wells was read at 540 nm by ELISA. All stages of the MTT assay and neutral red uptake were repeated five times, and the mean absorbance was considered mitochondrial activity, and the mean absorbance of neutral red cation was considered the rate of lysosome absorbance and cell membrane health. Cell proliferation in each dilution was determined by the above-mentioned equation.
3.5. Hepatocytes Apoptosis Analysis
3.5.1. Flow Cytometry
For detection of apoptosis, an Apoptosis Diagnostic Kit (annexin V-fluorescein isothiocyanate (FITC-PI) was used. The plasma membrane of the apoptotic cell can be structurally altered via exposure to phosphatidylserine on the cell surface (15). For this method, 106 hepatocyte cells were used and added to the microtubes containing DMEM with 10% FBS. Amaranth was then added to each microtube at concentrations of 200 μg/mL. Afterward, they were incubated at 24°C for a standard time of 24 hours. Subsequently, the tubes were centrifuged and washed three times with PBS. Then, a binding buffer was added and incubated for 20 min in the dark environment at RT (25°C). Finally, annexin V was added to the reaction medium. Cells were analyzed by flow cytometry using FACSCalibur flow cytometer (BD-Biosciences, USA) and Cellquest pro software (version 5.1).
3.6. Statistical Analysis
The control group was considered for all treatment groups, and its effect on cell survival was very scant and negligible. In this study, we used a cell line in two treatment groups with four doses obtained in the response dose process and a control group. All tests were assayed in triplicate for each treatment according to previous studies. Data were collected using a prepared checklist, and all data, including impact rate, growth time in the culture medium, and retention percentage, were statistically analyzed by SPSS20 software. Due to the fact that the study was an experimental study performed on a cultured sample, and in such studies, the disease is not evaluated, or a clinical condition is not performed in a specific population, the sample size was not considered. The cell line used was hepatocytes prepared from the cell bank of the Hydatid Cyst Research Center. In each test of 5,000, after calculating the mean of different experimental groups, the obtained values were analyzed by one-way ANOVA and then Newman-Keuls test. In all groups, the results were standardized as mean ± SD, and P < 0.01 was considered statistically significant.
4. Results
Hepatocyte cells were placed in four culture media containing amaranth extract with dilutions of 10, 50, 100, and 200 μg/mL and a control culture medium for 24 hours. The dilution of amaranth extract was accurately measured by laboratory methods, and it was added to eight culture media. The two culture media were control media to which no amaranth extract was added. After 24 hours, using cell survival tests, including MTT assay and neutral red, the percentage of cell growth and proliferation in five culture media, including four dilutions of the amaranth plant, was measured and compared with the control group (Figure 1).
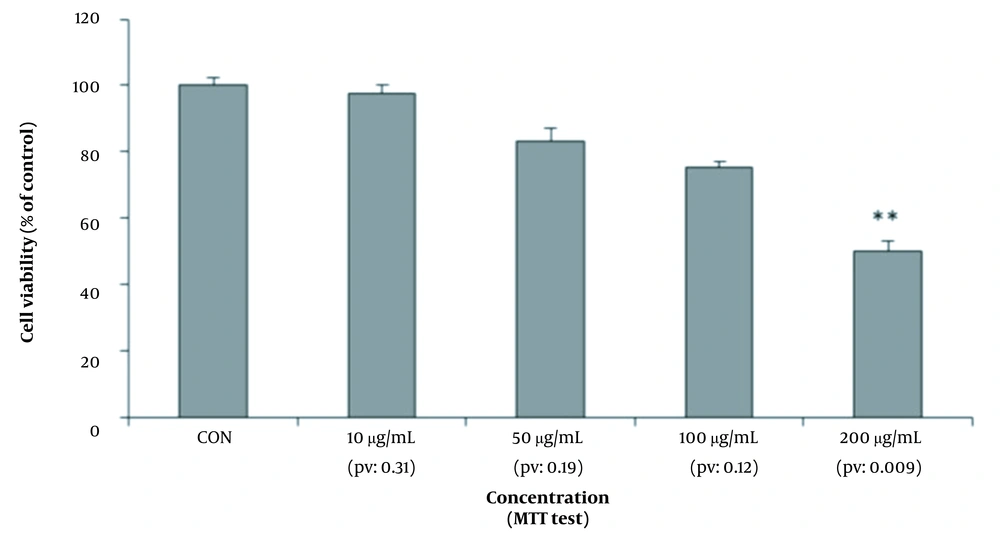
As shown in Figure 2, the survival rate of hepatocytes was measured by the MTT method in four culture media containing different dilutions of amaranth extract and compared with the control culture medium. According to this diagram, at concentrations of 10, 50, and 100 μg/mL, the survival of cells was not significantly reduced. At the concentration of 200 μg/mL (P-value = 0.009), cell survival decreased to less than half (40%). Therefore, amaranth had a dose-dependent cytotoxic effect and reduced cell survival, and this reduction was higher at higher doses.
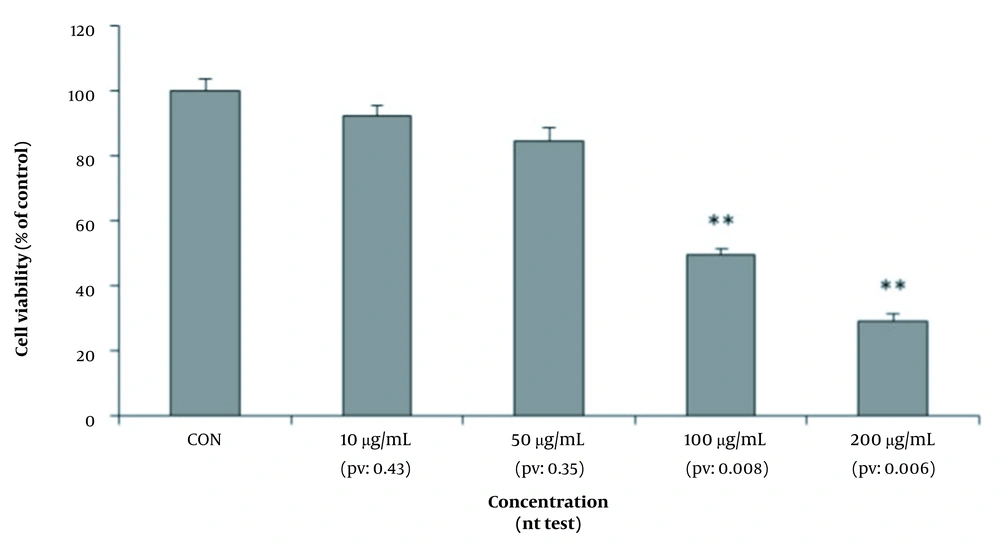
As shown in Figure 3, the survival rate of hepatocytes was measured by neutral red test in four culture media containing different dilutions of amaranth extract and compared with the control culture medium. The viability of the cells did not decrease significantly at concentrations of 10 and 50 μg/mL.
Cell survival rate was observed a half (50%) at the dilution of 100 μg/mL (P-value = 0.008), while cell survival rate was less than it (30%) at the dilution of 200 μg/mL (P-value = 0.006). This method also confirmed the dose-dependent cytotoxicity of amaranth.
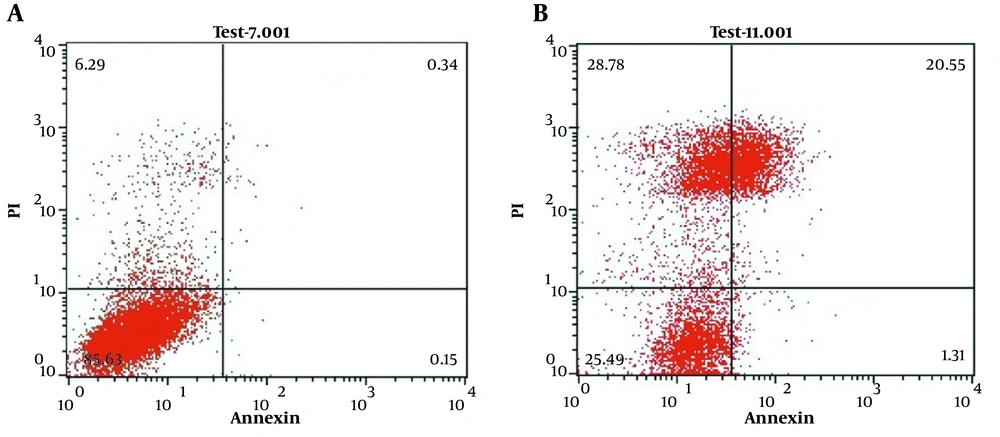
We used annexin-propidium iodide (PI) flow cytometry analysis to investigate the apoptotic effect of amaranth extract on hepatocyte cells. The results demonstrated that the amaranth extract had an apoptotic effect on hepatocytes at concentrations of 200 μg/mL. As shown in Figure 4B, the significant population of hepatocytes was positive for PI. It represents the cells were undergoing late apoptosis.
5. Discussion
The liver is the largest organ in the body and is the principal site of metabolic actions and detoxification. Liver damage is caused by drugs, toxic chemicals, and viral infections. The red dye obtained from amaranth is annually used in foods (such as soft drinks), sweet powder, candy, medicines (pill covers), and cosmetics (lipstick) in more than 60 countries. Various studies have shown that amaranth has many positive and negative effects on hepatocytes. Amaranth leaves are an excellent source of protein, fiber, squalene, anthocyanins, and tocotrienol.
Yawadio Nsimba et al. (10) and Zeashan et al. (9) showed that this plant had a high antioxidant capacity. Plate and Arêas also confirmed that the use of the amaranth plant reduced LDL and total cholesterol (7). Kabiri et al. showed that the consumption of a diet containing amaranth has a significant reduction effect on total cholesterol and C-reactive protein while increasing apolipoprotein A and HDL (8). Al-Dosari showed that oral administration of this substance reduced the serum levels of liver enzymes, bilirubin, and cholesterol, and the acute toxicity test showed no complications or mortality due to the use of this substance (11). Moreover, the study of Ashok Kumar et al. showed an increase in the hepatoprotective properties of this substance (13). Despite the widespread use of amaranth, few studies have been conducted on the toxic effects of this plant. However, in other studies, such as Collins and McLaughlin (5) and Baig (14), the toxic effects of this plant have been investigated. In previous studies, no fundamental study has been performed on the effects of amaranth toxicity on human hepatocytes. In clinical practice, we noticed that some people mistakenly take herbal medicines such as amaranth and suffer from acute liver failure. Therefore, we decided to evaluate the toxicity of this plant on the human hepatocyte cell line in the present experimental/interventional study.
Evaluation of mean absorbance as mitochondrial activity (conversion to formazan by mitochondrial reductase enzyme and purple dye) in MTT assay showed that the percentage of live hepatocytes significantly decreased during different stages by increasing the dose of amaranth extract so that cell survival reduced to less than half at a dose of 200 micrograms (Figure 4). In the neutral red test, during different stages, by examining the average absorbance of neutral red cation as the amount of lysosome absorbance and cell membrane health, it was proved that the amaranth plant had cytotoxic effects in different dilutions of 100 and 200 μg/mL and reduced the survival of hepatocytes (Figure 4). Therefore, it should be noted that consumption of amaranth, even at a concentration of 200 μg/mL, can lead to destructive effects on the liver, such as jaundice and acute liver failure.
The results of the present study are consistent with the studies of Collins and McLaughlin (5), and Baig (14). They showed that high doses of amaranth had systemic and toxic effects inconsistent with studies by Yawadio Nsimba et al. (10), Zeashan et al. (9), and Plate and Arêas (7). Our study is similar to the study conducted by Wang et al. for the evaluation of the hepatoprotective and hepatotoxic effects of rhubarb extract (a Chinese herbal medicine) at different doses in rats. There was a bidirectional effect of the herbal extracts on these rats at different doses, so there was a protective antioxidative property of the product at the low doses and hepatotoxic fibrosing effects at the higher doses (15). Such effects might exist at the amaranth plant extraction. Thus, it may be hepatoprotective at low doses with cautious consumption, such as the most previous studies, and may be hepatotoxic at higher doses and careless taking.
5.1. Conclusions
We found that the amaranth plant has toxic effects at toxic doses by reducing cell survival; so, the cell survival rate is reduced to less than half at a dose of 200 μg/mL, which can cause jaundice and acute insufficiency. Therefore, due to the widespread use of this plant, this issue should also be considered, and in addition to the benefits and positive effects of this plant, the hepatotoxic effects should also be noted, and arbitrary consumption of this plant should be limited.
![Control environment (A) and the effect of different doses [10 μg/mL (B), 50 μg/mL (C), 100 μg/mL (D), and 200 μg/mL (E)] of amaranth extract on hepatocytes’ viability Control environment (A) and the effect of different doses [10 μg/mL (B), 50 μg/mL (C), 100 μg/mL (D), and 200 μg/mL (E)] of amaranth extract on hepatocytes’ viability](https://services.brieflands.com/cdn/serve/3170b/64e7ba91994c64c198b350ba7e2b4f29ddc57398/zjrms-120348-g001-F1-preview.webp)