1. Context
A new human infectious virus with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) manifestations was reported to the World Health Organization (WHO) in late 2019. Severe acute respiratory syndrome coronavirus-2 was the cause of the Corona disease. As of 4 November 20121, over 247 million confirmed cases were confirmed, and over five million deaths have been reported (https://covid19.who.int/). It quickly progressed from an epidemic outbreak in Wuhan city, Hubei province, China, to a pandemic worldwide (1, 2). Previously two outbreaks caused by the coronavirus family of severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 and Middle East respiratory syndrome (MERS-CoV) in 2011 were experienced (1, 3). There are different hypotheses about the source of the origin of COVID-19 and its connection with the seafood market, suggesting animal-associated transmission (4, 5). Two molecules, including angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), have been associated with influenza and SARS-CoV and SARS-CoV-2. These molecules mediate viral entry into the affected cells (6). The major clinical features manifested by patients during the COVID-19 pandemic are related to the involvement of the respiratory system. Symptoms, including fever, cough, and respiratory difficulty, are the main complaints in around 80% of infected individuals with COVID-19 who experience a mild form of the disease. The clinical outcome also has a diverse range of infection severity from asymptomatic to septic shock or patients needing intensive care unit admission, mechanical ventilation, and death (7-9).
The hematology laboratory is one of the main informative sections for screening, diagnosing, and improving viral infectious diseases. Previously, the contribution of MERS-CoV infections with thrombocytopenia was confirmed (10). Several studies reported increased D-dimer and prothrombin time (PT) levels following SARS-CoV-1 infections (10).
2. Evidence Acquisition
This study was compiled using reports retrieved in PubMed, Google Scholar database, Islamic Science Citation, and Magiran databases from 2019 to 2021. The keywords included coagulation tests, PT, partial thromboplastin time (PTT), platelet (PLT) count, and coagulation. Hematologic changes play an essential role when dealing with viral infectious diseases. COVID-19-associated coagulopathy in venous thromboembolism (VTE), arterial thromboembolism, myocardial infarction (MI), cerebral infarction, and disseminated intravascular coagulation (DIC) is a predictor of mortality in this disease. Given the widespread role of COVID-19 in coagulation complications, it should be considered one of the treatment approaches. COVID-19 research is still in its early stages, and large-scale clinical studies are needed to determine the effect of SARS-CoV-2 on the coagulation and fibrinolysis system and to guide therapeutic development. Thus, we aim to report changes in PLT count and coagulation tests in COVID-19 patients. We hope this report provides helpful information for physicians, laboratory sciences, and researchers.
3. Results
3.1. Platelet Changes
Viral infections are usually associated with changes in PLT count and are not specific to COVID-19 patients (11). Evidence strongly supports thrombocytopenia as an important indicator and prognosis related to the severity and mortality of COVID-19 cases (11). In critically ill patients, thrombocytopenia is a common cause that is consulted by hematologists. So, it is usually difficult to assign this agent only to viral infection or, in particular, COVID-19 disease. In COVID-19, as a complicated and multi-organ damaged disease, a combination of various causes can be the reason for thrombocytopenia. Thrombocytopenia occurs in underlying liver disease, administered drug side effects, heparin-induced thrombocytopenia (HIT), primary hematological diseases, immune thrombocytopenia (ITP), thrombotic thrombocytopenic purpura (TTP), imminent DIC and viral infection (11). In a meta-analysis of nine studies with 1,779 COVID-19 participants, 399 (22.4%) patients had severe disease. It has been demonstrated that more severe forms were more common in COVID-19 with lower PLT count (95% CI, from 29 to 35 × 109/L). Subgroup analysis comparing patients based on survival showed that the lower PLT count was associated with mortality (95% CI, 39 to 57 × 109/L (n = 1427)) in patients with severe COVID-19, and the risk is more than five times higher (6). Only 8% of ICU and 4% of non-ICU patients had PLT counts of less than 100 × 109/L when they were admitted (12). Yin et al. compared PLT counts in COVID-19-associated acute respiratory distress syndrome (ARDS) patients with non-COVID-19 ARDS patients and found only slight clinical changes (215 ± 100 vs. 188 ± 98 × 109/L, P = 0.015) (13). Another study by Chen et al. reported that 12% of affected cases with COVID-19 experience thrombocytopenia. Another study showed that 36.2% of cases had thrombocytopenia with a PLT count of less than 150 × 109/L (14). However, the degree of decreased PLT count and its association with death rate has not been completely understood (14). In addition, the grade of decreased PLT count was mild in some COVID-19 cases. In contrast with COVID-19, some other viral diseases, such as Dengue, has been reported with more severe thrombocytopenia to < 40 × 109/L (15). A meta-analysis of nine studies showed that in severe COVID-19 patients, the decrease in PLT count was significantly more noticeable and independently related to higher mortality (16). Pseudo thrombocytopenia is rarely seen in COVID-19 patients. Platelet counts may be normal at admission, but there is a gradual reduction in PLT after a few days without signs of bleeding. The peripheral blood smear investigation showed PLT aggregate; PLT count became normal after re-sampling with citrate anticoagulant (10). Liu et al. studied the dynamic changes in platelet counts among patients admitted to hospital and demonstrated that monitoring platelet counts may have a predictive property for prognosis. As a result, thrombocytopenia on the first administration day was associated with three times higher mortality than those without thrombocytopenia (P < 0.05) (17). The degree of decreased PLT count was also related to survival, as evaluated using linear modeling (6). An analysis of monitoring the dynamic changes in the PLT count in 30 cases of COVID-19, along with various clinical and laboratory factors such as age and PLT to lymphocyte ratio (PLR), revealed that these parameters were related to the duration of hospitalization, the severity of the disease, and the worse outcome. Higher values of PLT count in the length of treatment were linked to longer durations of hospitalization, either with increased PLR (8). Two separate studies conducted by Qu et al. and Yang et al. demonstrated that a higher PLR could be a predictor marker in COVID-19 (18, 19). Zou et al. introduced a predictive model with 96.2% accuracy that relied on only two parameters, including PLT counts and hypoxemia (20). In an extensive retrospective study of 1,476 patients with COVID-19 by Yang et al., (6) thrombocytopenia was present in 10.7% of survivors vs. 72.3% of non-survivors (6, 8).
In a study of 189 COVID-19 patients who were hospitalized in the infectious diseases wards of Imam Reza hospital in Mashhad, Iran, the number of PLT significantly increased at the time of discharge (P < 0.05) (21). Another study on 225 COVID-19 patients admitted to Shariati hospital, which is a tertiary care university hospital in Tehran, Iran, showed at the time of admission, the mean PLT count was considerably lower in non-survived patients (P = 0.023). The platelet to PLR was linked to death, with non-survivors having a significant PLR than survivors (22).
3.1.1. Thrombocytopenia Mechanisms
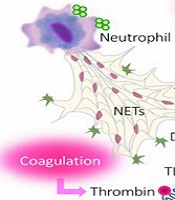
There are several reasons for thrombocytopenia, including the direct interaction of SARS-CoV-2 on PLT production, autoimmune destruction of PLTs by antibodies, and increased PLT consumption (10). Some mechanisms of PLT production inhibition are the direct effect of infection on bone marrow or PLT progenitors and precursors by virus invasion by binding to CD-13 receptors, which reduces thrombopoietin due to liver damage and high PLT consumption. They decreased their life span by destroying the PLTs (11, 23) (Figure 1).
Activation of platelets in COVID-19 patients (24)
Another research demonstrated that platelets express ACE2 and TMPRSS2. The SARS-CoV-2 spike glycoprotein binding to ACE2 on PLTs can activate alpha IIb/beta 3 and increase P-selectin expression, resulting in increased thrombosis. The elevated plasma thromboglobulin levels and PLT surface expression of P-selectin after angiotensin II infusion in healthy subjects supported PLT activation (25). The main pathomechanisms of COVID-19 coagulopathy are angiotensin II-induced coagulopathy, hyperfibrinolysis due to factor XIIa and Kallikrein-Kinin System (KKS) activation, and DIC, all elicit thrombosis, resulting in systemic inflammation, coagulation activation, and fibrinolysis. All of which are related to organ dysfunction, bleeding, and poor outcomes. COVID-19 coagulopathy can be improved by controlling thrombin, plasmin, and inflammation (25). Platelets have been demonstrated to directly destroy pathogens of invertebrates and indirectly dispose of infections by inducing neutrophil extracellular traps (NETs). Platelet factor 4 (PF4), a CXC chemokine generated from activated PLTs, is employed in the innate immune system to increase leukocyte responses (24). Also, PF4 is known as a heparin-binding protein and neutralizes heparan sulfate on the endothelium surface, reducing the antithrombotic capabilities of the endothelial surface. Platelet factor 4 binds to negatively charged bacterial surfaces to aid opsonization and interacts with IgG and its receptor Fc to excite PLTs, macrophages, and neutrophils (24). It is assumed that thrombocytopenia can be caused by increased PLT consumption due to the formation of microthrombi. Among COVID-19 patients, there is a trend toward higher immature reticular PLTs, indicating active cell production per megakaryocyte. This higher rate of PLT renewal leads to the release of young macrothrombocytes, which may account for the observed increase in the mean PLT volume (26) (Figure 1).
Global data show a correlation between severe vitamin D deficiency and coagulopathy in COVID-19. In severe COVID-19 patients, the hypercoagulable conditions may promote thrombosis in the lungs and other organs (27). PLTs play a key role in coagulation, inflammation, thrombosis, endothelium dysfunction, immune response, and bone metabolism. Thus, low vitamin D levels in COVID-19 patients are linked with a low PLT count, resulting in numerous PLT activation characteristics. It might indicate a greater risk factor for developing a more severe infection and lead to a more severe infection with higher risk of hypercoagulation (27). Vitamin D deficiency causes a rise in pro-inflammatory cytokines levels, such as tumor necrosis factor (TNF-α), interferon-γ (IFN-γ), and interleukin-6 (IL-6), which increases oxidative stress and accelerates megakaryopoiesis, in addition to activating PLT. This event causes the release of immature and activated PLTs from the bone marrow into the circulatory system, which increases and/or changes all PLT activation parameters, including mean PLT volume (MPV) and PLT distribution width (PDW) (27). Thrombocytosis was another unexpected finding in the patient, as most pediatric COVID-19 patients appear with a normal or low PLT count. Overproduction of pro-inflammatory cytokines, particularly IL-6, is a common cause of secondary thrombocytosis. In adults, it is substantially linked to the severity of COVID-19 (28). Altogether, three mechanisms, including reduced primary PLT production, decreased circulating PLT, and increased PLT destruction, lead to thrombocytopenia (Figure 2).
3.2. Coagulation Factors Changes
Prothrombin time and activated partial thromboplastin time (APTT) tests usually vary from normal or mild to moderate prolonged in COVID-19. Prolonged APTT has sometimes been reported to be due to the presence of heparin. Viral infections are usually associated with the temporary presence of lupus anticoagulant antibodies, which probably are a common cause of a prolonged coagulating test in COVID-19 cases (29). Decreased APTT is also reported in some cases, potentially because of the increase in fibrinogen and factor VIII as acute-phase proteins. At the same time, PT test may be increased in acute disease cases because of multiple organ failure beginning, including liver failure (12). A study with 183 coronavirus pneumonia showed that total mortality was 11.5%. The non-survivors demonstrated significantly increased D-dimer and fibrin degradation product (FDP) values and prolonged PT and APTT than survivors on the first day of hospitalization. In contrast, fibrinogen and anti-thrombin values were decreased significantly in non-survivors during hospitalization. D-dimer and FDP are increased in all non-survivors in the last days of hospitalization. It resulted from a wide coagulation activation, dysregulated thrombin generation, weakened natural anticoagulants, and fibrinolysis (7). A retrospective study was conducted on 1,061 patients who were admitted to Masih Daneshvari hospital in Tehran, Iran, with suspicious COVID-19. The median age of patients who died due to COVID-19 was 65. Coagulopathy was defined by high D-dimer values and prolonged PT, international normalized ratio (INR), and PTT (30). Lymphocyte ratio values were significantly lower inward patients than in those who died (31). Lymphocyte ratio values were significantly lower in ward patients than in those who died (31). In a study on 225 COVID-19 patients admitted to Shariati hospital, a tertiary care university hospital in Tehran, Iran, PT, and PTT were assessed in 182 patients’ blood tests at admission. Only 20.3% of the patients had PTT > 30 s), and 57.4% had prolonged PT (i.e., > 14 s). Patients with PT greater than 14 seconds were strongly associated with mortality (P = 0.0001) (22).
3.2.1. D-dimer
After the clot has formed, the fibrin mesh is broken down by the fibrinolytic system. The activation of the plasmin enzyme produces the D-dimer, which is made up of two D pieces of fibrin. This shows that fibrin is destroyed in the bloodstream. The D-dimer represents the activation of the coagulation and fibrinolysis systems. Technically, the amount of D-dimer in the body is measured using a monoclonal antibody and several commercial kits on the market (32).
As an important screening coagulation test, the D-dimer test is a technique to determine the soluble fibrin degradation product resulting from the ordered clot breakdown by the activated fibrinolytic system (33). Production of D-dimer relies on the in-ordered activation of three enzymes: Thrombin, factor XIIIa, and finally, plasmin (33). D-dimer test has various applications. It has been assessed to determine the optimal duration of anticoagulation in VTE patients, diagnose and monitor DIC, and help recognize COVID-19 patients at high risk for VTE (33). In a retrospective report of the epidemic in China of 1,099 hospitalized COVID-19 patients, 260 out of 560 patients (46.4%) showed increased D-dimer (≥ 0.5 mg/L), while changes were more marked in severe cases (59.6%) vs. non-severe cases (43.2%). D-dimer changes during the progression of the disease can reflect the prediction of severity, and their elevated levels are associated with worsening outcomes (34). Thrombocytopenia in 7% and elevated D-dimer in 32% of COVID-19 patients were seen more in non-survivors than survivors during the clinical course (35). D-dimer and fibrinogen elevations are commonly stopped in admitted-to-hospital cases, and higher levels are rarely seen with more complicated conditions (36). Cui et al. report a high incidence of coagulopathy and VTE in COVID-19 (37). It seems that respiratory worsening is related to coagulopathies. The values of fibrinogen and D-dimer characteristically elevate during the primary phase in COVID-19 patients. First-line coagulation screening tests, including PLT count, APTT, PT, are typically normal in this phase of the disease (12). Han et al. showed that D-dimer and FDP were higher in clinically severe diseases than in mild diseases. Thrombin time in definitive COVID-19 patients was also shorter controls (38). Gao et al. developed a cut-off level of 0.28 ng/L for D-dimer as a predictive marker for defining the severity of the disease (39). A study demonstrated treatment with unfractionated heparin and low molecular weight heparin to decrease the mortality rate in cases with abnormal hemostatic tests results (40). As a mechanism of viral invasion, the apoptotic processes due to injury to the endothelial cells of the vascular structure disrupted endothelial cells and triggered coagulation, thereby increasing D-dimer (41). Tang et al., in a retrospective study, compared the conventional coagulation tests of 183 COVID-19 patients. The mortality rate was 11.5% in whom D-dimer and FDP were significantly higher. Furthermore, PT and APTT were longer than in survivors (P < 0.05). Clinical signs of DIC were seen in 71.4% and 0.6% of non-survivors and survivors, respectively (42). Because FDP in DIC is more sensitive to D-dimer by increasing fibrinolysis, it may be a better alternative for determining the prognosis of COVID-19 (43).
In another study on 201 COVID-19 patients by Wu et al., prolonged coagulation results were associated with a significantly elevated risk of emerging ARDS and increased mortality. Prolonged PT was associated with ARDS, while increased D-dimer was associated with ARDS and death (44). Increased D-dimer was shown in 36% of cases in a survey of 99 COVID-19 patients by Chen et al. in Wuhan, China (14). The International Society on Thrombosis and Hemostasis (ISTH) diagnostic criteria for DIC was developed more commonly in non-survivors than survivors (71.4% vs. 0.6%, respectively) in median four days from hospitalization. Also, numerous critically ill COVID-19 cases have been shown to develop coagulopathy, anti-phospholipid immunoglobins, and elevated risk of arterial and venous thrombotic events, including cerebral infarction (7). In a study on forty-one COVID-19 patients, D-dimer was assumed to be a continuous variable case requiring ICU care presented with higher D-dimer values (mean = 2.4) vs. non-ICU cases (mean = 0.5) (P = 0.0042) (45). In another survey, 138 ICU patients presented higher D-dimer values (mean = 414) in comparison to non-ICU patients (mean = 166) (P < 0.001) (46). Virus attachment to endothelial cells does much endothelial damages. Injured endothelial cells are a trigger point for initiating PLT aggregation and activating coagulation cascade, and finally thrombosis formation and ischemia event (16). Another possible mechanism is that the virus-causing cytokine storm phenomenon may intercede endothelial injury through local inflammation and recruitment of the monocyte and other WBC, causing worsening local inflammation and cell injury, thus initiating PLT aggregation and thrombosis (34, 35). The inflammatory response in monocytes and macrophages has been linked to thrombin production, and the inhibition of thrombin activity can be a potential therapeutic approach (12). However, Han et al. showed decreased anti-thrombin activities in COVID-19 cases and normal activity in the control group (85% vs. 99% (P < 0.001), the activity maintained above 80%. Thus, the supplementation of anti-thrombin may not be necessary in most cases (38). Anti-thrombin replacement is often performed to treat sepsis-associated DIC in Japan when the level decreases to below 70% and the patients fulfill DIC criteria. It is unknown whether similar criteria are appropriate for COVID-19 cases. Concerning the other anticoagulants, diffidently decrease protein C and protein S levels and elevate the soluble thrombomodulin values are demonstrated in Dengue fever. Therefore, supplementation of these natural anticoagulants may be the choice for certain cases. However, no clinical studies have revealed its efficacy (47). Various guidance documents have suggested prophylactic anticoagulation with low-molecular-weight heparin (LMWH). Based on studies demonstrating decreased mortality among patients with severe COVID-19 or patients with D-dimer values greater than six-fold the upper limit of normal who received LMWH (40). Rare studies worked on thrombotic microangiopathy events, and new research is warranted to measure a potential interaction between COVID-19, von Willebrand factor (VWF), and ADAMTS-13. Many studies have focused on the thrombin generation responsible for the hypercoagulability of SARS-CoV-2; however, SARS-CoV-2-induced PLT hyper-reactivity needs more attention and research (48).
4. Conclusions
It was established that the PLT decreased in COVID- 19 cases to various degrees. The blood was in a hyper coagulation condition than in severe clinical circumstances. The PLR, PT, PTT, D-dimer, and FDP were correlated to disease severity in COVID-19 cases. Therefore, these predictor markers should consider managing the COVID-19 patients’ clinical treatment to understand critically worsened patients’ early identification and interference.