1. Context
Most grown-up organs hold a populace of somatic stem cells that can react to physiological conditions or injury by delivering new cells for tissue homeostasis or repair. The brain, for quite some time, was thought of as a special case; it was broadly expected that the grown-up cerebrum contained begetters to create glial cells; however, new neurons could just frame during embryonic development (1, 2). According to the World Health Organization, treating nerve abnormalities and injury is a difficult therapeutic issue. Regarding self-regeneration and mending, the human body has a certain character limit. The human nervous system has a finite capacity for self-rehabilitation and self-repair (3). Prolonged loss of motor function, which typically occurs following nerve injuries, imposes a considerable burden on patients. Injuries due to damage to the nervous system have a high financial cost for patients since the loss of capacity prompts some degree of incapacity that can be deep-rooted. Peripheral nerve injury is a common physical issue. Long-gap peripheral nerve repair is frequently postponed and rarely finished. Preparation of scar tissue and delayed neuronal growth rates may occur following these injuries (4-6). The architecture of the peripheral nerves in distal stumps is retracted independently when the peripheral nerve ruptures. Because the distal stump no longer receives nutrition from the neuron cell body, the protrusions and myelin sheaths degrade and finally vanish, but the proximal degeneration may be reversed (7, 8). Tissue engineering is about recovering, replacing, or repairing damaged tissues and organs. One of the essential elements of tissue engineering is the construction of permeable three-dimensional (3D) frameworks that create a pleasant environment for healing organs and tissues. Three-dimensional tissue engineering platforms are built using various construction methods and biomaterials (9, 10). Bioprinting can accurately scatter cell-loaded biomaterials to build complex 3D functioning living tissues, a 3D bio-fabrication breakthrough (11). As an added substance fabricating strategy, 3D bioprinting depends on the statement of biomaterials, either typifying cells or stacked with cells later on, on a micrometer scale to shape unobtrusive constructions equivalent to tissue. By and large, a three-hub mechanical stage controls the development of extruders to print the bioink in the necessary calculation and shape (12). In this review, we will study this technique’s applications in repairing the peripheral nerve system.
2. Evidence Acquisition
2.1. Nervous System Physiology and Anatomy
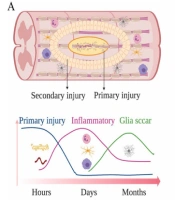
The human nervous system is a complex network containing many cells that control every physiological function. When it comes to nerve injuries, the intricacies of the neurological system provide a significant barrier to researchers. Glial cells are a permanent component of the nervous system. They help peripheral and central nervous system neurons maintain homeostasis, build myelin, and give support and assurance (3, 13). Spiral glia, astrocytes, astrocytes, and microglia each impact nervous system advancement. They are involved in neuronal birth, movement, axon detail, and development through circuit gathering and synaptogenesis (14). Schwann and satellite cells, both peripheral neurons, are glial cells in the peripheral nervous system. Like astrocytes in the central nervous system (CNS), satellite cells in the ganglia detach and support neurons (15). The cranial cavity and spinal cord protect the cerebrum and vertebrae. Some parts like the retina, olfactory, and optic nerves are considered part of the CNS. The cerebrum is divided into three parts in all animals: The cerebellum, cerebral hemispheres, and brain stem. The frontal cortex, which is split into four lobes, is the most important part of the brain. The cerebellum comprises a white matter core and a gray cortex on the outside. The brain stem connects the frontal cortex to the spinal cord and is the extra part of the brain. It’s far from an unpredictable tangle of nerve tissue that controls basic activities like respiration, perception, and heartbeat. The spinal cord connects to the brain stem, which runs inside the rigid vertebral section. It is made up of a gray matter core surrounded by white matter. In a cross-segment view, the gray matter exhibits a butterfly pattern. Separately, axons enter and exit it through the dorsal and ventral ganglia. These roots, which are part of the PNS, work together to form the spinal nerves. Axons transporting information into the spinal cord are found in the dorsal root, while axons that transfer information away from the spinal cord are found in the ventral root. Neurons, glia, endothelium, and meningeal cells are eliminated after a severe injury to the CNS structure, which leads to Wallerian degeneration. The PNS is made up of ganglia and cranial nerves, which come from the spinal cord and brainstem. The skin and muscles are innervated by peripheral nerves, which allow the CNS to transmit and receive electrical impulses from the skin and muscle (Figure 1) (16). Holes or layers like the brain and spinal cord do not shield peripheral nerve fibers. Axon breakage and myelinated strand degeneration are caused by fringe nerve injury, and axotomized neurons might die. The Schwann cells (SCs) divide, and their sheaths are lost when the myelin sheath, which maintains the axon intact by providing critical contact, is damaged. Over a month and a half, the lack of axon connection animates and activates macrophages, leading them to phagocytose myelin debris and axon filaments. The absence of a link also energizes SCs, causing them to proliferate and move to the injured region. Recovery containers are segments of SCs and fibroblasts that are clustered together. The recovery process begins with a period of brain reorganization designed to restore cell respectability. Neurotrophic factors (NGF and BDNF) are key in the recruitment of neuritis (3, 17).
Physiological changes at the injury site following A, SCI; and B, PNI (16)
2.2. Microanatomy of Peripheral Nerves
The anatomy of the peripheral nerve must be understood to improve the design of nerve guidance conduits (NGCs). To successfully promote axonal regeneration and functional recovery, NGCs should offer a scaffold structure of the epineurium or perineurium and establish a milieu comparable to that of the extracellular matrix (ECM). Endoneurium, perineurium, and epineurium are the three layers of connective tissue in the peripheral nerve ECM from the inside out. The endoneurium is the connective tissue layer on the axon’s surface and myelinated axon fibers are axons wrapped with SCs. The endoneurium and the basal lamina of SCs contain the ECM, an important nervous system component (18). The diameter of axons at different nerve locations ranges from 0.5 to 20 μm. The sensory end-organs and muscle fibers are connected via the endings of myelinated and nonmyelinated axon fibers. The epineurium is the outer connective layer, whereas the perineurium is the connective layer that covers the surface of the epineurium’s many nerve fascicles. The blood arteries supply peripheral nerve axons that supply the epineurium, perineurium, and endoneurium (19). The perineurium and endoneurium’s closely linked microvessels have a high permeability for material movement and exchange. These microvessels form the protective blood-nerve barrier (BNB) and play an important function in the stability of the surrounding nervous system milieu (20).
2.3. Bioprinting Technology
Three-dimensional printing, also known as additive manufacturing or fast prototyping, is a method of layer-by-layer assembly of items using a sequence of cross-sectional slices. It’s like chopping a potato into sliced, shredded, chopped, and mashed pieces, then 3D printing them together to ensure their integrity. These four ways of assembling potatoes correlate to four common 3D printing processes: Planar projection is utilized in digital light processing (DLP), filaments are used in fused deposition modeling (FDM), microspheres are used in inkjet printing, and powders are sintered in selective laser sintering (SLS) (21, 22). The most frequently utilized bioprinting innovations include inkjet printing, expulsion printing, laser-aided printing, and stereolithography (3, 11, 23, 24).
2.4. Bioprinting with an Inkjet Printer
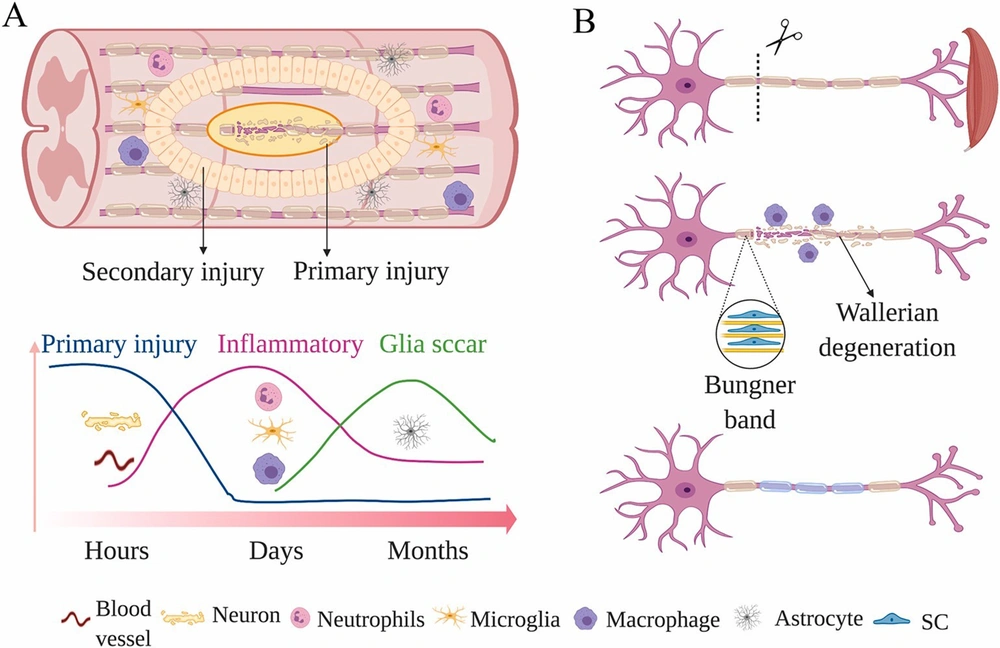
Inkjet technology was initially used to present the concept of in situ bioprinting in 2007. This approach attempts to heal and recreate damaged tissues with curved surfaces or even more complex geometries, whereas traditional 3D printing techniques are usually limited to a flat substrate. However, this technology’s safety and aseptic characteristics must be thoroughly verified before being used in clinical settings (23). Liquid bioink has been successfully produced using inkjet technology for bioprinting; controlling these variables leads to further restrictions in the printing process (7). Inkjet-based cell printing is a non-contact approach that uses a pressure pulse controlled by a thermal bubble or piezoelectric actuator to discharge droplets of cells or biomaterials (Figure 2) (25). Inkjet-based bioprinting method delivers 2D and 3D patterns by layering picoliter droplets of biomaterials onto a substrate (26, 27). Inkjet has sharp images, and investigations have shown that inkjet can induce the creation of small brain networks by precisely placing cells (7).
The printing mechanics of major cell printing methods. A, Micro-extrusion-based cell printers extrude materials from a syringe needle using a computer-controlled piston or pneumatic pressure; B, To make droplets out of liquid solution, inkjet printers utilize various methods (thermal bubble, piezoelectric, or electromechanical valve); C, The energy of a concentrated laser beam is utilized to produce localized heat, which is then utilized to make a liquid droplet (25).
2.5. Laser-assisted Bioprinting
Laser-helped 3D bioprinting (LAB) chips away at the standards of laser-initiated forward move. LAB is more uncommon than inkjet or microextrusion bioprinting, yet its applications for tissue and organ designing are consistently expanding (26). The image polymerization technique is the most popular procedure for laser-assisted bioprinting of biomaterials. In this cycle, many cells can be imprinted and cell functionality maintained (11, 26). LAB, which is superior to other bioprinting procedures, can store cells at a thickness of up to 108 cells/mL with the goal of individual cells for every drop utilizing a high-velocity laser beat (26, 28, 29).
2.6. Extrusion Bioprinting
Extrusion bioprinting fabricates scaffolds from temperature-controlled polymerized materials using contact printing. Because it produces a high amount of cell loss, this printing technology is often used to print acellular materials. Extrusion bioprinting is used to deposit cells into spherical shapes on occasion. This approach should not need high printing clarity and is much more likely to be a pouring operation than a printing procedure. Furthermore, this strategy makes regulating single cells difficult, which is important for neuron regeneration or creating functioning tissues with a higher degree of cell organization of certain anatomic characteristics (30).
2.7. Stereolithography
In the early 1980s, stereolithography was created as a solid free-form production technology. The term “STL” stands for stereolithography, and the STL file format began as a native file format from Computer-Aided Design (CAD) software since it was the first 3D printing technology. The majority of 3D printers and newer bioprinters now support the STL format. This STL file is sliced to generate G-code, which is used to program the STL machine. The system’s main components are a reservoir filled with a photocurable polymer solution or resin, an x-y-axis controlled laser, and a production stage with z-axis control. STL is a laser-assisted additive manufacturing technology that includes employing an ultraviolet (UV) laser to photopolymerize the surface of a photosensitive polymer solution. The stage is progressively lowered, enabling layers to polymerize on top of one another and construct 3D structures from the bottom up (31). Traditionally, STL was used to create cell scaffolds, but it is now used to print bioink with cells (32, 33).
2.8. Bioprinting Process
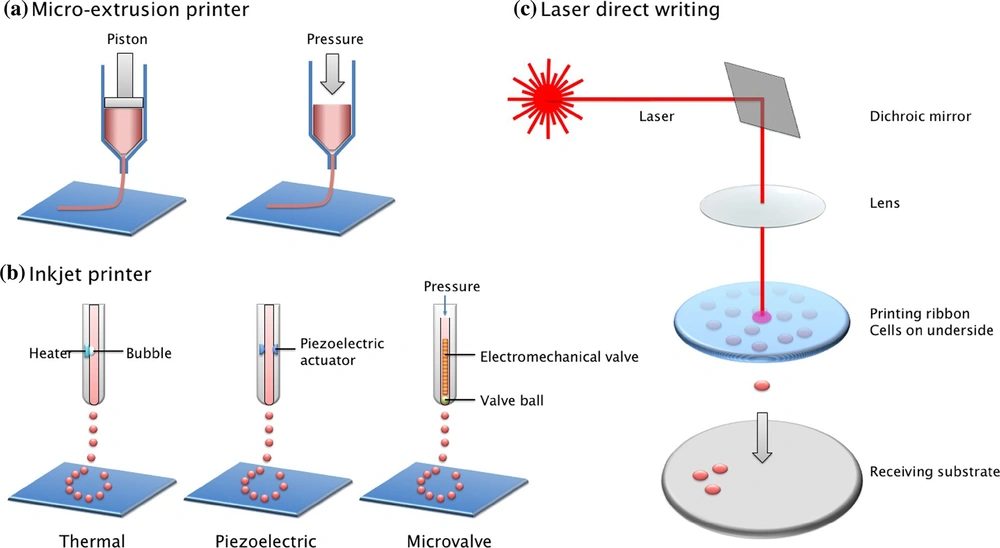
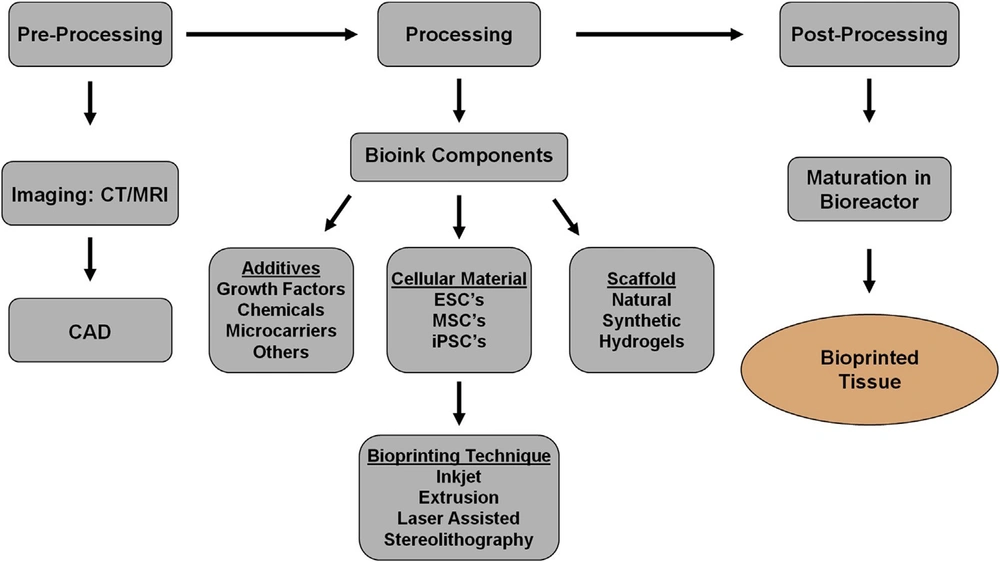
Three-dimensional bioprinting, the automated, computer-aided deposition of cells, biomaterials, and biomolecules, is now possible because of recent advances in engineering, material science, computer science, and cell biology. Layer-by-layer biological materials are deposited using software that drives the printer hardware (34, 35). Bioprinting is a procedure that includes digital design, material selection, and pre-and post-processing. The created pictures are transferred to bioprinting equipment, and the bioinks are loaded for processing in the first step. The printed structures are placed in a bioreactor to mature into tissues (Figure 3) (36, 37). The next sections go through the specifics of each of these three phases.
An overview of bioprinting is depicted in the diagram below (38).
2.8.1. Pre-processing
A 3D model of a tissue or organ may be employed for computer design. Computer-assisted design is a method of obtaining information on the tissue’s structure and composition. The objective is to produce a 3D model of the structure. MRI and CT scans can be used to build this type of model. The most common method of obtaining medical pictures is CT scanning. CT is non-destructive and repeatable when compared to other imaging methods. It also allows users to measure crucial biological characteristics quantitatively. The benefit of utilizing computational models is that they may assist in defining rules, predicting the characteristics of printed tissues, and improving implant design. The second strategy is to employ computer-assisted approaches. Bio-CAD systems are capable of simulating 3D anatomic structures, distinguishing between different tissue types, and generating the necessary computational tissue models. Bio-CAM manufacturing process viability may be predicted by simulating appropriate tissue models on computers. This method can also help us better understand the physical and chemical elements throughout the printing process. When Bio-CAD and Bio-CAM are used together, printing may be sped up, and the quality of the tissue generated can be improved. During this step, the biomaterial parameters should also be verified (27).
2.8.2. Processing
An appropriate bioprinter is utilized to print bioinks into required structures throughout the processing step. It is critical to use the best bioink possible to ensure a smooth printing process by evenly filling the bioink within the cartridge. The bioink flowability determines the printing capability. As a result, bioink characteristics have a significant impact on bioprinting outcomes.
2.8.3. Post-processing
This step’s main objective is to give the bioprinted tissue time to develop before being transplanted into the host. Bioreactors may provide a dynamic environment for tissue maturation while also assisting in the scaling up of bioprinting. Bioreactor conceptual design is presented to increase tissue fusion, maturation, and remodeling more effectively. The bioreactor has three perfusions, each with its unique function. Bioreactor intended to provide an intravascular perfusion system in the intra-organ branching vascular system. The third perfusion has several robust, porous, non-biodegradable, detachable minitubes. Temporal support and artificial microchannels are provided by these minitube (11, 36).
2.9. Materials Used in Bioprinting
Bioprinting produces usable tissue by printing natural frameworks made up of cells, growth factors, biomaterial platforms, and other components. The best biomaterials should be able to improve the arrangement of the surrounding host tissue and avoid the necessity for subsequent surgical operations to remove the embedding. Furthermore, it must be adequately robust to withstand an implant. The qualities of printed biomaterials utilized in tissue design should facilitate cell attachment, development, motility, multiplication, and separation (39).
2.9.1. Biomaterials in Bioinks
Printability and crosslink ability, mechanical characteristics, biocompatibility, side effects, and corruption controllability are all important pre-polymer features (38).
2.9.2. Natural Polymers
Gelatin, collagen, alginate, chitosan, hyaluronic acid (HA), and agarose are common polymers utilized as natural materials. In mammalian tissues, collagen is the most prevalent ECM component. The printed gel’s mechanical strength and the cell-loaded hydrogel’s maintainability may be inadequate (40, 41). A gel grid frame can be made by adding bivalent particles, such as Ca2+ or Mg2+, to improve the mechanical strength of the structure. When NaOH is employed as a cross-linker, chitosan, a polysaccharide with a straight structure, may quickly frame a gel grid. Decellularized extracellular matrix (dECM) might be used as a bioink since it is a regular inferred material. The dECM has all of the elements and complexity of a conventional ECM. As a result, the bioprinted dECM structure is perfect for reproducing the printed cells’ microenvironment, resulting in enhanced cell morphologies and capabilities. Despite the high cell feasibility, the bioprinted dECM platform was able to fully realize cell-cell collaboration and tissue utility. A variety of tissues, including adipose, ligament, and heart tissue, have previously been studied using adapted dECM. Long-haul work reservation applications show promise for these bioprinted tissue analogs. Bioprinting is also commonly done using composite natural materials. Inkjet printing created cardiovascular tissue growth using an alginate and gelatin composite filled with cardiomyocytes. Expulsion printing was utilized to shape the heart valve using a similar composite hydrogel. A printed heart valve was brooded in calcium chloride for 10 minutes to show cross-connection. A new bioprinting platform increased the cell’s practicality by 82 percent. Alginate and collagen hydrogel was used to frame vascularized bone tissues, while chitosan was used to print vessels that resembled cell microfluidics (27).
2.9.3. Synthetic Polymers
One of their main benefits is that the chemical and mechanical properties of the polymers generated are reasonably predictable. Furthermore, synthetic polymers often surpass natural polymers in terms of mechanical properties. On the other hand, manufactured polymers give essentially no specialization in cell capacity. Calcium phosphate (CaP), tricalcium phosphate (TCP), hydroxyapatite (HA), polylactic acid (PLA), polyglycolic acid (PGA), poly (lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL) are all bioprinting materials. PLA, PGA, and PCL are all biocompatible, biodegradable, and mechanically strong materials. Biomimetic signals like peptides or cement proteins may be used to establish physiologically realistic cell-biomaterial interactions in a poly (ethylene) glycol (PEG) hydrogel and its derivatives (42). CaP/PCL, CaP/PLA, and CaP/PEGDA are some of the composites that have been studied for bone regeneration. These synthetic polymers hasten the interplay of bone repair while reducing aggravation and unfamiliar body dismissals. It is commonly utilized in bone development and healing. TCP can increase primary compressive strength and offer excellent osteoconductivity when employed to make hard tissue. However, a framework built with pure TCP is far too fragile to be considered. By adding PLA, the compressive strength may be increased. TCP/PLA platforms printed with a combination of TCP and PLA showed enhanced soundness and induced osteoblast migration. Because of its amazing regenerating ability for bone tissues in vivo, HA is perhaps the most widely used composite in both exploration and facilities. Bioprinting has also been used to create hard tissues using HA/PCL and PLGA/TCP/HA composites (11, 43).
2.9.4. Tissues and Cells
Tissue and cell printing technologies are improving, and one day they may completely remove the requirement for allogenic tissue implantation or novel mechanical devices. Heart cells, osteoblasts, pluripotent stem cells, endothelium cells, fibrosarcoma cells, and osteosarcoma cells were used to construct bioprinted tissue types. Many characteristics must be addressed during cell selection and bioprinting preparation to make the tissue that closely matches the actual thing. While working with bioprinted cells, the microenvironment should mimic physiological conditions in vivo to stimulate their in vivo work. Several cell types are needed to serve specialized tasks in the desired tissues. When many kinds of cells are printed for a complex tissue development process, they may be produced simultaneously in separate locations or in different types of hydrogels. The combining of tissues is an important step in the formation of planned tissue. The capacity to separate features of the cells is required for this interaction. The phrase “heterotypic cell combination” refers to grouping comparable cell types. Dendritic cells from the bone marrow are mixed with neurons from the cerebrum or myocyte cells from the heart. This interaction can boost cell development. The printed cell structure may shrink, and mechanical strength may be diminished due to the combined marvel effects. Legitimate platform support might help to prevent some undesirable misshapen. The connections between cells and the platform also influence the character of the printed products. Finding the right platform is almost as easy as finding the right cell source. The tissue-creating substance should be biocompatible with the tissue type in question, allowing cell attachment and dissociation while reducing corruption. It should also aid in the development of innervation and vascularization (11, 20).
2.10. Applications of Bioprinting in Nerves System Regeneration
More than a billion groups all throughout the planet are thought to experience the ill effects of nervous system issues. Constant degenerative illnesses or horrendous injuries of the nervous system influence CNS work. Neurodegenerative sicknesses, because of maturing, are additionally getting progressively significant. Despite extensive studies, no medications that may restore neural function are now available. Despite their low cost, ease of maintenance, and flexibility to various cell types, 2D cultures are extensively used. They cannot sustain the cell-cell and cell-ECM connections that occur in vivo (44). The ability of various 3D tissue engineering frameworks to mix various cell types and build sophisticated brain tissue connection topologies has been investigated. This section examines 3D neural tissue models created using 3D bioprinting (9). Current treatments for nerve recovery inside harmed tissues have had limited accomplishment because of confounded neural life systems and inhibitory obstructions in situ. Progress in 3D bioprinting has enabled scientists to create innovative 3D frameworks with complicated architectures to overcome the challenges that plague solid and characterized brain tissue regeneration. Three-dimensional bioprinted frameworks have the amazing benefit of being extremely changeable, which increases a more prominent similarity to the local natural engineering of in vivo frameworks, which is one of a few possible neuroregenerative therapies approaches that are being investigated nowadays. 3D bioprinting allows the creation of mind-boggling and many-sided structures on the micrometer scale. Brain tissue can be 3D printed to create complex mathematical circulations, including various cell types, biomaterials, and development variables that may be altered for patient treatment (3, 14).
3. Conclusions
Regenerative medicine and tissue design research have been centered on the recovery, replacement, or reclamation of damaged functional living tissues and organs for many years. Tissue transplantation, both autograft and allograft, is frequently used to treat serious damage or chronic disease, especially as the population ages and the danger of injury or sickness grows, resulting in a reduction in tissue engineering and capacities. In any case, contributor lack is consistently a genuine test, and an incredible number of patients pass on while sitting tight for appropriate organ transplantation. Significant costs and allogeneic transfer, which results in resistive dismissals, are also major roadblocks for recipients. Tissue engineering has been viewed as a viable answer to the pressing demand for organs. Because of the complicated tissue engineering, it is necessary to combine a variety of phone kinds and elements in a definitive manner to create a functional 3D design. Three-dimensional bioprinting, often known as organ printing, has the potential to achieve these goals. The technology is still in its infancy, with several obstacles to overcome. However, bioprinting remains the most promising method of dealing with the development of practical organs to relieve the strain of organ deficiency.