Introduction
Colorectal cancer is the third most prevalent cancer. It is the third leading cause of cancer-related deaths in Iran [1]. The origin of CRC is due to accumulation of genetic and epigenetic abnormalities in the intestinal epithelium, which may lead to the development of colorectal adenocarcinoma. Other tumors (10-15%) originate from a microsatellite instability (MSI) pathway which is characterized by the mismatch repair (MMR) genes leading to failure for DNA mismatches repair during replication and generation of inserted or deleted bases [2-5]. This phenomenon caused novel alleles detectable as a change in allele size between tumor and normal DNA. Microsatellites are simple DNA sequences which consist of a repeating unit of 1-5 bp with 25-60 bp in length and are commonly in the form of CAn [6-8]. Expansion or contraction of these sequences due to increase or decrease in the repeat units gives rise to what is referred to as MSI [9]. MSI is the important underlying event in 85-90% of hereditary [10-12]. In sporadic CRC in which MSI is supposed to be associated to 10-15% of malignancies [10, 13-15].
It has been argued during a second consensus workshop held at the end of 2002 that the original microsatellite panel has limitations resulting from the inclusion of dinucleutide markers, which are less sensitive and specific for detection of tumors with mismatch repair deficiencies. Among the suggested changes was the exclusive use of mononucleutide repeats improving the sensitivity of MSI detection in CRC. In this study we used five quasimononucleutide NCI markers including BAT-25, BAT-26, NR-21, NR-24, NR-27, are used as a pentaplex in several recent studies [13-17].
Microsatellite instability-high (MSI-H) is characterized by presence of MSI in more than 40% (two of five markers) examined microsatellite instability-low (MSI-L) by presence of fewer than 40% of markers and microsatellite stable tumors (MSS) by absence of any MSI, in markers examined. The aim of this study was to determine the incidence of each mononucleutide marker in colorectal patients, comparison of frequency of five markers in panel and their sensitivity in detecting MSI tumors. In the current study panel of biomarkers was assessed in the sporadic colorectal patients and the clinicopathological associations were evaluated from all over the Iran, from Taleghani hospital in Tehran.
Materials and Methods
Sample collection and DNA preparation: In this experimental study the patients were selected by pilot method. The study groups were 80 consecutive originally Iranian patients which were histologically confirmed as colorectal cancer and impulsively enrolled in the study. Demographic and clinicopathological data of each patient including age of onset, location of tumor were collected from their medical records and directly by contacting the patients and their family. The patients with positive family history of cancer such as patients with Lynch syndrome, familial cancer and FAP (familial adenomatous polyposis) were excluded from the study.
| Size | Gene | Genbank number | Repeat | Primer sequences | Amplicon size (bp) |
|---|---|---|---|---|---|
| Inhibitor of apoptosis protein-1 | AF070674 | 27 A 5′UTR | F: AACCATGCTTGCAAACCACT R:CGATAATACTAGCAATGACC | 87 | |
| SLC7A8 | XM 033393 | 21 T 5′UTR | F: GAGTCGCTGGCACAGTTCTA R: CTGGTCACTCGCGTTTACAA | 109 | |
| Zinc finger 2 | X60152 | 24 T 3′UTR | F: GCTGAATTTTACCTCCTGAC R: ATTGTGCCATTGCATTCCAA | 131 | |
| c-kit | X06182 | 25 T intron 16 | F: TACCAGGTGGCAAAGGGCA R: TCTGCATTTTAACTATGGCTC | 153 | |
| hMSH2 | U04045 | 26 A intron 5 | F: CTGCGGTAATCAAGTTTTTAG R: AACCATTCAACATTTTTAACCC | 183 |
Primer Sequences for MSI Assay
A written consent form was obtained from the patients. The study was approved ethically by the research center of gastric cancer and liver disease board, Shahid Beheshti University. Tumoral DNA was obtained from 80 cancerous tissues by Qiagen kit. Normal DNA was extracted from peripheral blood of individuals.
From an ongoing study on MSI in sporadic CRC, 80 specimens were analyzed. Fluorescent PCR products were analyzed by capillary electrophoresis using an ABI3100 genetic analyzer (applied biosystem, Belgium) and genemapper software 3.7 for interpretation purposes. MSI at two or more loci was defined as MSI-H, instability at a single locus was defined as MSI-L, and no instability at any of the loci was defined as microsatellite stability (MSS).
Microsatellite instability assay: The MSI analysis was performed on paired tumoral DNA from cancerous tissues and genomic DNA from whole blood, using 5 loci in the panel (NR-27, NR-21, NR-27, BAT-25 and BAT-26).MSI carriers were identified by analysis of tumor tissue using polymerase chain reaction according to the recommended panel.
DNA extracted from tumor and normal tissues used as template in separate PCRs by using a panel of microsatellite markers for MSI testing [18]. PCR was carried out in 20 μL reactions containing 1 μL DNA, 0.2 μL Taq polymerase (5 U/μL), 0.4 μL dNTP (10 mM), 0.5 μL of each primer, 2 μL 10× reaction buffer and 1.6 μL MgCl2 solution (25 mM). The primer sequences are presented in the table 1. Amplification was performed in a thermocycler which consisted of one cycle of 95°C for 5 min, 40 cycles of 95ºC for 45 s, 54°C for 45 s and 72°C for 1 min, followed by a final extension of 72°C for 30 min. PCR products were subjected on 2% agarose gels and stained with ethidium bromide for visualizing the bands.
Statistical analysis: Statistical analysis was performed using the SPSS-16 software. The correlation between two variables was evaluated using Pearson’s χ2 and Fisher’s exact test when required. Regression analysis was performed to rank the factors associated with MSI and identify the predictors of MSI. Statistical significance was defined as p<0.05.
Results
A total of 80 sporadic colorectal cancer patients including 50 (62.5%) men and 30 (37.5%) women were enrolled in the study. The mean age of patients was 58 years in males and 53 years in females (ages ranged between 25 and 86).
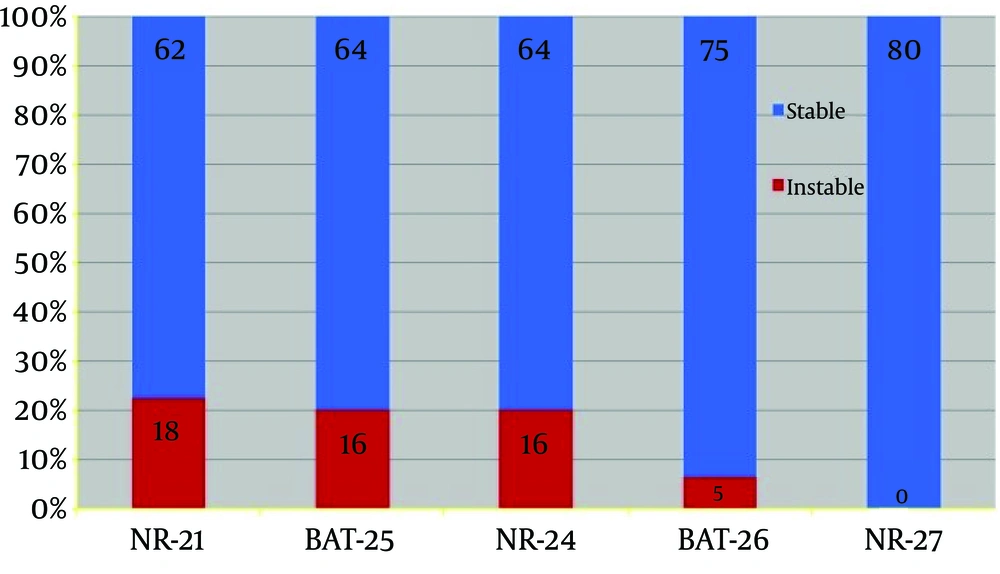
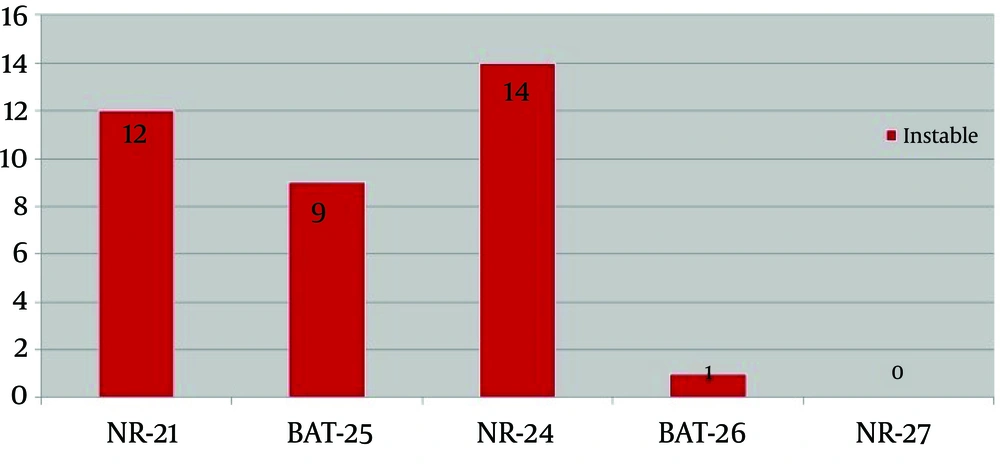
MSI analysis: A representative of MSI analysis is shown in figure 1. Microsatellite instability was detected in 36 of 80 cases (45%) with colorectal cancer. MSI analysis revealed that 17 cases of MSI-H (21%), 19 MSI-L (23%) and 44 MSS (55%). Figure 1 represents MSI analysis of the studied markers in studied patient’s samples. Instability is observed in the tumoral DNA compared to the DNA from the normal DNA sample. The most instable markers were NR-21, NR-24 in which instability was detected in 45% of patients. Among 17 MSI-H patients, instability in NR-24 occurred in 14 cases, NR-21 in 12 cases, BAT-25 in 9 cases, BAT-26 in 1 case, and NR-27 does not revealed instability (Fig. 2). Among 17 MSI-H patients, instability of two markers was detected in 60% patients, 23% patients had instability in three markers, 11.2% patients had instability in 4 markers and instability of all the markers was observed in 5.8% patients.
Clinicopathological data: Although the overall percentage of males was greater in the studied population (50 vs. 30%) the rate of MSI-H in females was close to male, (47.1 vs. 52.9%), with no statistical significance. In the MSI-H group, the mean age was 54.3 years whereas in MSL-L and MSS groups, it was 51 and 50 years respectively. All of 69.4% of tumors were in the colon, 30.6% tumors were in the rectum; among MSI-H patients. Significant correlation between MSI and location of the tumor was not observed.
Discussion
In this research the most instable markers were NR-21 and NR-24 in which instability was detected in 45% of patients. Iran is a cancer prone region, in which the incidence gastrointestinal cancer including CRC is above the average [1-5]. In order to better clarify the genetic features of CRC and its clinical rele¬vance in this high risk area, we studied MSI, as an important genetic pathway of CRC carcino¬genesis, for the first time in this aspect. The frequency of microsatellite instability in sporadic colorectal cancer is estimated to be 15-20% and in patients with HNPCC up to 85% [13-17]. In the present study, MSI was detected in 45% of patients and 21% of them having MSI-H phenotype which is higher than the previous reports [5], suggesting that the molecular epidemiology of genetic alterations, involved in the CRC carcinogenesis, has different patterns in the Iranian population. Among all markers NR-21 had the highest sensitivity with 23% and BAT-26 was least detected. BAT-25 (20%) and NR-24 (20%) were second and third instable markers, respectively.
Previously 170 sporadic CRCs in Iran were analyzed, with two MSI markers, BAT25 and BAT26, and reported 19.4% MSI-H [5]. We observed that the rate of MSI-H in females is very close to males, although the proportion of males was greater than females among the patients, inconsistent with the trend in Iran, previously reported by Mousavi et al. [12]. Various studies demonstrated that using mononucleotide markers without applying dinucleotide markers were highly effective for determining the MSI-H status of CRCs [18-20]. In 2011 Montazer-Haghighi et al. were analaized 80 HNPCC Iranian patients by this panel, the results were compatible with our findings that NR-21and BAT-25 were of the most frequent markers [21, 22]. It is believed that different genetic pathways involved in CRC carcinogenesis are associated with different environmental exposures and thus, introduces a divergent accumulation of risk factors and related genetic alterations in a different subset of patients [21].
It was suggested that mononucleotide repeats improving the sensitivity of MSI detection in CRC. The use of mononucleotide repeat microsatellites for MSI characterization has been shown to be advantageous over many dinucleotide microsatellites due to the quasimonomorphic nature of both loci and their high sensitivity to MSI [22, 23]. The NCI proposed the revised Bethesda guideline criteria for CRC screening [24, 25]. However, the current standard method of MSI analysis using five quasi mononucleotide markers is still time-consuming and expensive. Furthermore, microsatellite markers are examined in DNA amplified from tumor and normal tissues, and amplification might be difficult due to the limited amount of available tissues and DNA [25].
The aim of the current investigation was to find the most promising mononucleotide MSI marker to detect sporadic colorectal cancer. Several investigations have been examined frequency of MSI markers like as the study in Iran which demonstrate that the frequency of NR-21 and BAT-25 was considerable as a diagnostic tool for CRC, which was in relation to our study results [23]. Our results demonstrated that NR-24 marker have the most instability in sporadic colorectal cancer while in contrast to two groups that reported that BAT-26 and BAT-25 have the highest sensitivity and specificity in the similar panel in HNPCC patients [24-27]. Furthermore, another study support the previous results was performed on the pentaplex panel demonstrated that the fidelity each marker as follow 100% for BAT-26, 96.9% for BAT-25, 87.5% for NR-24, 97.0% for NR-21, and 97% for NR-27 nonetheless, the results were in contrast of our finding. In a new approach 5 quasimonomorphic markers including BAT-25, BAT-26, NR-21, NR-22, and NR-27 in Slovenian population tested. Their finding revealed that NR-21 was the most polymorphic marker and their finding was compatible with our results [28-30].
High frequency of MSI suggests consideration of MSI testing to determine the more sensitive and effective methods prior to chemotherapy in our population. It seems that among 5 mononucleotides markers NR-21, BAT-25 and NR-24 are the most instable markers. Therefore using a triplex panel including 3 mentioned MSI markers should be more usful markers for identifying MSI status in patients with sporadic colorectal cancer. We need more accurate MSI testing to perform and improve prognostic and predictive panel for colorectal cancer in other populations.