1. Background
Intra-ventricular hemorrhage (IVH) commonly affects premature newborn infants (1). It is among the most frequent causes of death and cerebral damage in low birth-weight preterm newborns (2). A classification for IVH based on ultrasound was developed in 1984 (3). The grading of hemorrhage as I - IV is as follows: grade I: when hemorrhage is limited only to the germinal matrix, grade II: intra-ventricular hemorrhage without ventricular dilatation, grade III: IVH with ventricular dilatation, and grade IV: IVH associated with intra-parenchymal hemorrhage.
The worldwide incidence of IVH ranges from 3.70 to 44.68% (2). It occurs in 50% of cases on the first day of life and in 90% of cases on the first third days of life (4). The incidence of IVH varies based on gestational age and birth weight. The average incidence of IVH in preterm infants with a gestation age of 22 to 28 weeks is 32% (5).
The determinants of IVH in preterm infants are different, including immaturity of the germinal matrix (a highly functional metabolic region with intense angiogenesis) (6). The increased permeability of the blood-brain barrier (BBB) results in the crossing toxic substances into the brain. Coagulation disorders with higher bleeding tendency and genetic traits are sometimes present (7). Disruption of cerebral homeostasis and blood flow occurs by risk factors, including oxidative stress, infection, twinning, placental retro hematoma (8), altered cerebral autoregulation (9), patent ductus arteriosus (10), variations in systemic blood flow caused by respiratory distress at birth, using positive pressure ventilation for the management of respiratory distress, and the presence of metabolic acidosis (11). Coagulation disorders are found in preterm infants with IVH and could increase bleeding risk (12).
IVH can be associated with ventricular dilatation and white matter lesions that result in brain atrophy (13). The risk of cerebral palsy is increased in preterm infants with severe IVH, especially infants with a birth weight of less than 1000 g (14). IVH is responsible for post-hemorrhagic hydrocephaly in up to 15% of preterm infants due to the impaired desorption of cerebrospinal fluid (CSF) (15).
2. Objectives
The present study aimed to describe the outcomes of premature infants with IVH at 6 and 12 months of age and assess the risk factors of adverse outcomes and the relationship between the age of IVH occurrence and neurodevelopmental outcome.
3. Methods
This prospective longitudinal study was conducted on preterm infants with a gestation age of less than 37 weeks in the North West of Iran from September 1, 2019, to October 30, 2020. Infants with major congenital malformations, severe birth asphyxia (first-minute Apgar score less than 4), persistent hypoglycemia, severe hyperbilirubinemia, exchange transfusion, and inborn errors of metabolism were excluded. Preterm infants with ultrasound evidence of IVH were included in this study. The Ethics Committee of Tabriz University of Medical Sciences approved this study.
Gestational age was estimated by the last menstrual period and first-trimester ultrasound findings. The estimated age was confirmed by the Ballard score (16). Intra-ventricular hemorrhage was diagnosed by transmontane ultrasound on the first 3 - 5 days, then weekly until days 28 - 30 by a pediatric radiologist. Ultrasound examination was repeated weekly in patients with IVH. We categorized IVH into two groups: IVH in the first week of life and delayed IVH, defined as IVH diagnosed at serial brain ultrasound examination after the first week of life.
The neurodevelopmental examination was routinely performed at the corrected age of 6-12 months using the Ages and Stages Questionnaire (ASQ) in the Neonatal Follow-up Centre. The Persian version of the ASQ was used in this study, validated by Sajedi et al. in Iran (17). This questionnaire assessed the child's development in five areas: Communication, gross and fine motor skills, problem-solving, and personal/social skills. When patients did not meet at least two of the five domains, the ASQ was considered abnormal and defined as suboptimal psychomotor development. Patients with suboptimal neuromotor function were referred to an experienced developmental pediatrician or pediatric neurologist who was unaware of IVH and its grade.
We performed statistical analyses using the statistical package for social sciences (SPSS) version 16.0. The quantitative data were presented as mean ± standard deviation (SD), and the qualitative data were shown as frequency and percent. An independent student’s t-test analyzed continuous, normally distributed data. The categorical data between the groups were compared using the chi-square or Fisher’s exact tests. A P-value less than 0.05 was considered statistically significant.
4. Results
During the study period, 40 infants were diagnosed with IVH, of whom 4 cases died during the hospital stay, and IVH was grade IV in 3 of them. Twenty-three neonates (63.9%) were boys, and 24 cases (66.7%) were delivered by cesarean section. Seven infants (19.4%) were twins, and 3 cases (8.3%) were triple pregnant. Mothers in 19 cases (52.8%) received antenatal corticosteroids.
The studied patients' mean birth weight and gestation age were 1361 ± 564 g and 29.3 ± 2.3 weeks, respectively.
The IVH grade was I in 13 (36.1%), II in 11 (30.6%), III in 6 (16.7%), and IV in 6 (16.7%) patients. The mean duration of hospital stay after birth was 34.6 ± 22 days. Mechanical ventilation was used in 11 neonates (30.6%). There was a history of tension pneumothorax and chest tube insertion in 2 patients (5.6%). Four neonates (11.1%) developed post-hemorrhagic hydrocephaly.
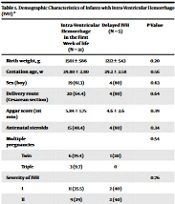
The IVH occurred in the first week of life in 31 neonates (86.1%) and after 2 weeks of life in 5 patients (13.9%) (delayed IVH). The demographic characteristics of the studied patients are shown in Table 1. The severity of IVH was determined by ultrasound examination, and grades II and I were the most common in both groups.
| Intra-ventricular Hemorrhage in the First Week of Life (N = 31) | Delayed IVH (N = 5) | P-Value | |
|---|---|---|---|
| Birth weight, g | 1501 ± 566 | 1212 ± 543 | 0.20 |
| Gestation age, w | 29.80 ± 2.80 | 29.2 ± 2.58 | 0.56 |
| Sex (boy) | 19 (61.3) | 4 (80) | 0.63 |
| Delivery route (Cesarean section) | 20 (64.4) | 4 (80) | 0.64 |
| Apgar score (1st min) | 5.84 ± 1.75 | 4.6 ± 2.6 | 0.39 |
| Antenatal steroids | 15 (48.4) | 4 (80) | 0.34 |
| Multiple pregnancies | 0.54 | ||
| Twin | 6 (19.4) | 1 (20) | |
| Triple | 3 (9.7) | 0 | |
| Severity of IVH | 0.76 | ||
| I | 11 (35.5) | 2 (40) | |
| II | 9 (29) | 2 (40) | |
| III | 6 (19.4) | 0 | |
| IV | 5 (16.1) | 1 (20) |
Demographic Characteristics of Infants with Intra-ventricular Hemorrhage (IVH) a
Associated risk factors of IVH showed in Table 2. Seizures occurred in eight (25.8%) cases in infants with IVH in the first week of life. Hydrocephalus developed in four (11.1) patients; all were in IVH in the first week of life.
| IVH in the First Week of Life (N = 31) | Delayed IVH (N = 5) | P-Value | |
|---|---|---|---|
| Mechanical ventilation | 10 (32.3) | 1 (20) | > 0.999 |
| Surfactant replacement therapy | 29 (93.5) | 5 (100) | > 0.999 |
| Pneumothorax | 2 (6.5) | 0 | > 0.999 |
| Hospital stay, d | 33.74 ± 22.26 | 40 ± 22.36 | 0.20 |
Associated Problems in Patients with Intra-ventricular Hemorrhage (IVH)
ASQ scores were evaluated in infants aged 6 - 12 months. The mean ASQ score at six months of age was 44.84 ± 10.36 and 44.00 ± 6.51 in the first week and delayed IVH groups, respectively (P = 0.76). The mean ASQ score at 12 months of age was 47.90 ± 9.10 and 48.00 ± 2.73 in the first week and delayed IVH groups, respectively (P = 0.72).
The neurodevelopmental delay was detected at six months after birth in 14 infants (45.1%) with IVH in the first week and two cases (40%) with delayed IVH (P = 0.37). Neurodevelopmental delay was diagnosed at 12 months after birth in eight infants (25.8%) with IVH in the first week and one patient (20) with delayed IVH (P = 0.17). Neurodevelopmental delay at six and 12 months after birth was not significantly different among patients with IVH in the first week (P = 0.18) and also among patients with delayed IVH (P > 0.999). Neurodevelopmental delay was significantly more common in patients with more severe IVH.
5. Discussion
In our study, IVH in the first week of life was more common than delayed IVH (three-quarters of cases occurred in the first week). Grades I and II of IVH consisted of 2/3 of IVH cases. The risk factors for IVH, including gestation age, birth weight, and respiratory distress syndrome needing surfactant replacement therapy and pneumothorax, were not significantly different among patients with IVH in the first week of life or delayed IVH. Approximately half of the infants with IVH had a neurodevelopmental delay at the corrected age of 6 months, which decreased to a quarter at the corrected age of 12 months; all of the patients with developmental delay had grade III or IV of IVH. Although 4 infants (12.9%) with IVH in the first week of life developed hydrocephalus, it was not progressive and no cases needed intervention. Radic et al. reported the prevalence of hydrocephalus to be 21% in patients with IVH, and they found no increased risk of morbidity in IVH patients associated with hydrocephalus (18). Another study showed a slight increase in the risk of developmental impairments in infants with IVH without white matter damage (19). They reported an association between developmental impairment and post-hemorrhagic hydrocephalus, similar to some studies (15, 20). Bolisetty et al. reported moderate to severe neurosensory impairment (18.6%) in 296 extremely preterm infants with grades I-II of IVH (21).
In the study by Min et al., grades III and IV of IVH were more common in the first week of life (22). The risk of cognitive, language, achievement performance, long-term neurologic and neurodevelopmental disability, seizures, and cerebral palsy increased in preterm infants with IVH grades III and IV, with or without complications (21, 23). The severity of IVH was not significantly different among patients with IVH in the first week of life or delayed IVH in our study. Neurodevelopmental delay at 6 and 12 months of age was detected in all infants with grade IV of IVH and 50% of infants with grade III of IVH. We found no case of neurodevelopmental delay in patients with grade I or II of IVH. In a study, patients with slight hemorrhage did not develop handicaps but had lower scores on tests assessing visual-motor integration (24). In a meta-analysis, the neurodevelopmental outcome was poorer in preterm infants with mild IVH than those without hemorrhage. Cerebral palsy and cognitive delay were more common in severe but not mild IVH (5).
Postnatal nursing care improvement may be significant in decreasing the risk of IVH. Some studies have recommended the middle position of the head and head elevation for improved cerebral venous outflow and avoidance of elevation of the legs during diaper change (25, 26). Neonatal individualized developmental care (NIDCAP) has been routine in our center since six years ago.
In our cases, IVH diagnosis was made by ultrasound examination. Associated peri-ventricular leukomalacia can be missed, especially in patients with ventricular dilatation, with complex periventricular white matter visualization.
Other limitations of this study are findings obtained from a single-center study with a limited number of infants included and short-term follow-up duration. Thus, future multi-center studies with large numbers of patients, complementary imaging with MRI to detect white matter involvement, and long-term follow-up for school-age performance assessment are recommended.
In conclusion, more severe stages of IVH are associated with neurodevelopmental delay in preterm infants in both IVH in the first week of life and delayed IVH, and prevention of preterm birth is a necessary intervention to improve neurodevelopmental outcomes.