1. Background
Celiac disease or gluten-sensitive enteropathy is a chronic inflammatory systemic disease of autoimmune origin in genetically predisposed individuals (1). Celiac disease affects approximately 1 - 2% of the general population of all races and countries, with a homogeneous distribution worldwide, and diminishes with age (2, 3). Celiac disease leads to important morbidity but very low mortality (4).
Celiac disease can be diagnosed at any age (5). The disease is seen in both children and adults, and most individuals consider the disease in children and adults to be basically the same (4, 6). Pediatric celiac disease is characterized by predominantly digestive characteristics with a typical malabsorption syndrome associated with digestive disorders (7). Nevertheless, adult celiac disease is presented in atypical forms characterized by extra digestive symptoms (5, 8).
The current diagnostic strategy for celiac disease is based on clinical suspicion and confirmed by serological and histological tests. However, the screening and diagnosis of celiac disease are relatively well-established (9, 10). The treatment of celiac disease is essentially based on a gluten-free diet that remains the only effective treatment (11).
2. Objectives
This study aimed to describe the main differences and similarities of celiac disease according to age and highlight the clinical features, diagnosis, associations of the disease, complications, and response to a gluten-free diet in infants, children, and adults.
3. Methods
A cross-sectional observational study was conducted on 223 patients diagnosed with celiac disease in the Pediatrics Department of the Oran University Hospital Center and the Gastroenterology Department of Tlemcen University Hospital Center (Western Algeria). The patients’ recruitment was carried out at the level of (1) the Pediatrics Department of the Oran University Hospital Center; (2) the Gastroenterology Department of Tlemcen University Hospital Center; and (3) the National Association of Celiac Protection in the Sidi Bel Abbes. Three groups were selected according to age, including infants < 2 years, children or adolescents ≥ 2 years, and adults ≥ 17 years. The current epidemiological survey was conducted over 2 years (2019 - 2021). The data were collected from the records of celiac patients and, according to the inclusion criterion, a histological and/or serological diagnosis of the disease with full respect for the confidentiality of the data and patients. All celiac cases lost to follow-up were excluded. The aforementioned criteria were applied to both genders and all age categories.
3.1. Statistical Data
The description, processing, and analysis of statistical data in the studied sample were carried out via SPSS software (version 22.0; Statistical Package for the Social Sciences, IBM Corporation; Chicago, IL. August 2013). Moreover, the data were presented in rates and cross-tabulations. The results were expressed as frequencies, means, and standard deviations. A Pearson’s chi-square test (χ2) was used to evaluate clinical symptoms and diseases associated with celiac disease according to the age group, and ANOVA test to evaluate an anthropometric parameter according to the age group. The statistical level of significance was P < 0.05. The reference values for the comparison between age groups were made using a 95% confidence interval.
4. Results
Among the 223 celiac patients enrolled in this study, 40 (17.9%), 109 (48.9%), and 74 (33.2%) subjects were infants < 2, children ≥ 2, and adults ≥ 17 years, respectively. There was a predominance of female gender with a gender ratio of 1.62. The average age was 15.17 ± 15.76 years in all patients, with 1.68 ± 0.38, 7.04 ± 3.68, and 34.44 ± 12.66 years in infants, children, and adults, respectively. A significant difference was noted in this regard (P < 0.001). The mean values of age at diagnosis and age of gluten-free diet were 10.79 ± 10.72 and 9.23 ± 9.93 years in all patients, respectively. A significant difference was observed in this regard (P < 0.001).
The mean body mass index (BMI) in the studied sample was 15.79 ± 2.47 kg/m2. The mean BMI values of children, adults, and infants were 15.13 ± 1.99, 16.74 ± 3.03, and 15.86 ± 1.90 kg/m2, respectively, with a significance of P < 0.001 in all age groups. The average hemoglobin level at diagnosis in all patients was 10.63 ± 1.89 g/dL, with 10.66 ± 1.89, 10.66 ± 2.00, and 10.52 ± 1.73 g/dL in children, adults, and infants, respectively, with no significant difference between the three age groups (P = 0.92) (Table 1).
| Characteristics | All Patients (n = 223) | CI 95% | Infants (< 2 Years) (n = 40) | CI 95% | Children (≥ 2 Years) (n = 109) | CI 95% | Adults (≥ 17 Years) (n = 74) | CI 95% | P-Value | P-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 15.17 ± 15.76 | 13.09 - 17.25 | 1.68 ± 0.38 | 1.56 - 1.81 | 7.04 ± 3.68 | 6.34 - 7.74 | 34.44 ± 12.66 | 31.51 - 37.38 | < 0.001 b | < 0.001 c (infants vs. children); < 0.001 c (infants vs. adults); < 0.001 c (adults vs. children) |
| Age at first diagnosis (y) | 10.79 ± 10.72 | 9.37 - 12.20 | 03.37 ± 3.93 | 2.11 - 4.63 | 6.53 ± 3.77 | 5.81 - 7.24 | 21.07 ± 12.49 | 18.18 - 23.97 | < 0.001 b | 0.68 (infants vs. children); < 0.001 c (infants vs. adults); < 0.001 c (adults vs. children) |
| Age of gluten - free diet (y) | 9.23 ± 9.93 | 7.92 - 10.54 | 1.80 ± 0.68 | 1.58 - 2.02 | 5.94 ± 3.37 | 5.30 - 6.58 | 18.09 ± 12.52 | 15.19 - 20.99 | < 0.001 b | 0.08 (infants vs. children); < 0.001 c (infant vs. adults); < 0.001 c (adults vs. children) |
| BMI (kg/m2) during the diagnosis | 15.79 ± 2.47 | 15.47 - 16.12 | 15.86 ± 1.90 | 15.25 - 16.47 | 15.13 ± 1.99 | 14.75 - 15.51 | 16.74 ± 3.03 | 16.03 - 17.44 | < 0.001 b | 0.197 (infants vs. children); 0.114 (infants vs. adults); < 0.001 c (adults vs. children) |
| Hb (g/dL) during the diagnosis | 10.63 ± 1.89 | 10.38 - 10.88 | 10.52 ± 1.73 | 9.97 - 11.08 | 10.66 ± 1.89 | 10.30 - 11.01 | 10.66 ± 2.00 | 10.19 - 11.12 | 0.92 | 0.916 (infants vs. children); 0.943 (infants vs. adults); 0.998 (adults vs. children) |
Abbreviations: BMI, body mass index; Hb, hemoglobin; CI, confidence interval; SD, standard deviation.
a Values are expressed as mean ± SD unless otherwise indicated.
b Significant level of analysis of variance (P < 0.05).
c Significant level of Tukey’s post-hoc test.
The symptomatological diversification assessment showed that the clinical features of the disease were defined as digestive disorders (n = 109, 48.9%) in all patients, with 56 (25.1%), 29 (13.0%), and 24 (10.8%) subjects in children, adults, and infants, respectively. The most common digestive disorder noted in the children group (≥ 2 years) were chronic diarrhea, abdominal bloating, abdominal pain, and vomiting in 59 (54.1%) (P = 0.004), 35 (32.1%) (P < 0.001), 64 (63.3%) (P = 0.026), and 39 (35.6%) (P = 0.183) patients, respectively. However, vomiting was frequent in infants (< 2 years) with 52.5%. Nevertheless, abdominal distension and epigastralgia were the most frequent digestive disorders noted in adults, with a frequency of 19 (25.7%) (P = 0.001) and 3 (4.1%) (P = 0.442) patients, respectively.
Weight loss (52.7%), anemia (51.4%), and asthenia (37.8%) were the main extra digestive disorders in the adult group, with statistically significant differences of P = 0.026, P = 0.008, and P = 0.004, respectively. However, stunting was the most extra digestive disorder noted in the children group, with a frequency of 32.1% and a significant difference of P = 0.016. Nonetheless, in the infant group, anemia was the most frequent extra digestive disorder in 39% of the patients (Table 2).
| Characteristics | All Patients (n = 223) | Infants (< 2 Years) (n = 40) | Children (≥ 2 Years) (n = 109) | Adults (≥ 17 Years) (n = 74) | P-Value |
|---|---|---|---|---|---|
| Digestive disorders | |||||
| Chronic diarrhea | 142 (63.7) | 33 (82.5) | 59 (54.1) | 50 (67.6) | 0.004 b |
| Abdominal bloating | 55 (24.7) | 17 (42.5) | 35 (32.1) | 3 (4.1) | < 0.001 b |
| Abdominal pain | 142 (63.7) | 19 (47.5) | 64 (63.3) | 54 (73.0) | 0.026 b |
| Constipation | 28 (12.6) | 6 (15.0) | 19 (17.4) | 3 (4.1) | 0.024 b |
| Vomiting | 90 (40.4) | 21 (52.5) | 39 (35.8) | 30 (40.5) | 0.183 |
| Abdominal distension | 30 (13.5) | 3 (7.5) | 8 (7.3) | 19 (25.7) | 0.001 b |
| Epigastralgia | 06 (2.7) | 0 (0.0) | 3 (2.8) | 3 (4.1) | 0.442 |
| Extra digestive disorders | |||||
| Anemia | 87 (39) | 12 (30) | 37 (33.9) | 38 (51.4) | 0.026 b |
| Anorexia | 23 (10.3) | 6 (15.0) | 11 (10.1) | 6 (8.1) | 0.511 |
| Asthenia | 56 (25.1) | 7 (17.5) | 21 (19.3) | 28 (37.8) | 0.008 b |
| Staturo-ponderal delay | 56 (25.1) | 11 (27.5) | 35 (32.1) | 10 (13.5) | 0.016 b |
| Weight loss | 90 (40.4) | 19 (47.5) | 32 (29.4) | 39 (52.7) | 0.004 b |
| Pallor | 63 (28.3) | 12 (30.0) | 25 (22.9) | 26 (35.1) | 0.191 |
| Weakness and muscle cramps | 23 (10.3) | 1 (2.5) | 6 (5.5) | 16 (21.6) | < 0.001 b |
| Depressed state | 5 (2.2) | 0 (0.0) | 1 (0.9) | 4 (5.4) | 0.075 |
| Skin rash | 13 (5.8) | 3 (7.5) | 2 (1.8) | 8 (10.8) | 0.035 b |
| Splenomegaly | 2 (0.9) | 0 (0.0) | 0 (0.0) | 2 (2.7) | 0.131 |
| Oral aphthosis | 7 (3.1) | 2 (5.0) | 2 (1.8) | 3 (4.1) | 0.530 |
a Values are expressed as No. (%).
b Significant.
The comorbidity of celiac disease in the patients was noted in this study. An anemia rate of 16.5% was observed in the children group. Nevertheless, a hypothyroidism rate of 8.1% was noticed in the adult group. Other autoimmune diseases were also noted in the adult group as Crohn’s disease (5.4%) and lupus (0.9%), with a significant difference (P = 0.001). However, allergy was present in the infant group with 22.5% (Table 3).
| Characteristics | All Patients (n = 223) | Infants (< 2 Years) (n = 40) | Children (≥ 2 Years) (n = 109) | Adults (≥ 17 Years) (n = 74) | P-Value |
|---|---|---|---|---|---|
| Comorbidity | 0.001 b | ||||
| Allergy | 15 (6.7) | 9 (22.5) | 6 (5.5) | 0 (00.0) | |
| Anemia | 28 (12.6) | 8 (20.0) | 18 (16.5) | 2 (2.7) | |
| Asthma | 4 (1.8) | 1 (2.5) | 3 (2.8) | 0 (0.0) | |
| Type 1 diabetes | 5 (2.2) | 1 (2.5) | 4 (3.7) | 0 (00.0) | |
| Lupus | 2 (0.9) | 0 (00.0) | 0 (00.0) | 2 (0.9) | |
| Crohn’s disease idism | 4 (1.8) | 0 (00.0) | 0 (00.0) | 4 (5.4) | |
| Osteoporosis | 2 (0.9) | 0 (0.0) | 1 (0.9) | 1 (1.4) | |
| Hypothyro | 13 (5.8) | 2 (5.0) | 5 (4.6) | 6 (8.1) |
a Values are expressed as No. (%).
b Significant at P < 0.05.
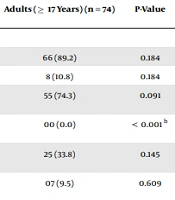
The results of serological tests of all patients with positive disease serology were 90.0% and 80.7% in infants and children, respectively, versus 89.2% in the adult group. No significant difference was recorded with serology (P = 0.184). This study also determined differences in the frequency of immunoglobulin G (IgG) at anti-transglutaminase (a-tTG) refers to assay positivity (P < 0.001) for all age groups. However, the immunoglobulin A-type (IgA) assay positivity was dominant in all the age groups.
The histological examination (at the time of diagnosis) according to the degree of severity of the lesion of the intestinal mucous membrane showed that 45.3% were grade partial villous atrophy. It was predominant in adults at 48.6% vs. 47.5% and 42.2% in infants and children, respectively. The total grade was dominant in adults (18.9%). There was no significance between the three age groups (P = 0.097) (Table 4).
| Characteristics | All Patients (n = 223) | Children (< 2 Years) (n = 40) | Children (≥ 2 Years) (n = 109) | Adults (≥ 17 Years) (n = 74) | P-Value |
|---|---|---|---|---|---|
| Serological tests | |||||
| Positive | 190 (85.2) | 36 (90.0) | 88 (80.7) | 66 (89.2) | 0.184 |
| Negative | 33 (14.8) | 04 (10.0) | 21 (19.3) | 8 (10.8) | 0.184 |
| IgA a-tTG assay positivity (U/mL) | 161 (72.2) | 29 (72.5) | 77 (70.6) | 55 (74.3) | 0.091 |
| IgG a-tTG assay positivity (U/mL) | 43 (19.3) | 15 (37.5) | 28 (25.7) | 00 (0.0) | < 0.001 b |
| IgA AGA assay positivity (U/mL) | 86 (38.6) | 21 (52.5) | 40 (36.7) | 25 (33.8) | 0.145 |
| IgA EmA assay positivity (U/mL) | 20 (9.0) | 05 (12.5) | 08 (7.3) | 07 (9.5) | 0.609 |
| Histological tests | 0.097 | ||||
| Partial to subtotal | 15 (6.7) | 05 (12.5) | 10 (9.2) | 00 (0.0) | |
| Partial | 101 (45.3) | 19 (47.5) | 46 (42.2) | 36 (48.6) | |
| Subtotal | 69 (30.9) | 11 (27.5) | 36 (33.0) | 22 (29.7) | |
| Subtotal to total | 02 (0.9) | 00 (0.0) | 00 (0.0) | 02 (2.7) | |
| Total | 36 (16.1) | 05 (12.5) | 17 (15.6) | 14 (18.9) | |
| Gluten-free diet adherence | 0.001 b | ||||
| Good adhesion | 141 (63.2) | 25 (62.5) | 81 (74.3) | 35 (47.3) | |
| Poor adhesion | 82 (36.8) | 15 (37.5) | 28 (25.7) | 39 (52.7) |
Abbreviations: IgA, immunoglobulin A-type; a-tTG, anti-transglutaminase antibodies; IgG, immunoglobulin G-type; AGA, antigliadin antibodies; EmA, endomysial antibodies.
a Values are expressed as No. (%).
b Significant at P < 0.05.
The gluten-free diet with good adherence was noted in 63.2% of all patients, with 62.5% and 74.3% in infants and children, respectively, versus 47.3% in adults. There was a significant difference in this regard (P = 0.001).
5. Discussion
The current study analyzed the differences between pediatric and adult celiac disease, and the obtained results showed that pediatric and adult celiac disease is characterized by a female predominance in all age groups with a gender ratio of 1.62. This predominance was also suggested by other studies (12, 13). In the present series, the mean age of celiac patients was 15.17 ± 15.76 years. The mean age at disease diagnosis was 10.79 ± 10.72 and 21.07 ± 12.49 years in children and adults, respectively. This issue is confirmed by other studies (14), as the disease can be diagnosed at any age. Therefore, the starting age for the gluten-free diet was 9.23 ± 9.93 years in all patients and 1.80 ± 0.68 years in infants. This suggests that the development of the disease is related to the cessation of breastfeeding and the early introduction of cereals containing gluten, confirmed by Poddar’s study results (6).
The mean BMI in the current sample was 15.79 ± 2.47 kg/m2; all the patients were malnourished (BMI < 16.5 kg/m2). This finding is similar to the findings of other studies conducted on Indian children indicating that growth retardation was a frequent manifestation of the disease (6). In a comparable way to adults, the present study’s results demonstrated an increase in the rate of BMI (> 16.5 kg/m2) comparable to a study by Dickey and Kearney (15), who showed that few celiac patients were underweight at diagnosis.
The clinical symptomatology of the disease was characterized by classic forms that are defined as digestive disorders, which were predominant in children (69%), mainly chronic diarrhea (54.1%), abdominal pain (63.3%), and abdominal bloating (32.1%), with a significant difference (P = 0.004; P = 0.026, and P < 0.001, respectively), which is consistent with a study by Rodrigo-Saez et al. (16) who noted that chronic diarrhea was the main feature of the disease in children. On the other hand, adults also expressed chronic diarrhea as the main symptom of the disease. The current study’s results are confirmed by Sood et al.’s study (17), in which chronic diarrhea was present in adults, in contradiction with other studies’ results (18). Therefore, the present study’s results underline that extra digestive manifestations are quite common in adults with celiac disease, such as weight loss (52.7%), anemia (51.4%), and asthenia (37.8%), with a significant difference (P = 0.004, P = 0.026, and P = 0.026, respectively). The current study’s results are suggested by several compliance studies (6). Although weight loss was the principal extra digestive manifestation (P = 0.004) in the infant group, the stature weight delay was dominant in children (P = 0.016). The aforementioned data are similar to those of Vivas et al.’s study (18).
In the present study, there were many diseases associated with celiac disease in children and adults; however, in the adult group, Crohn’s disease and hypothyroidism seem to be more frequent, as noted in the results of studies by Doya et al. (19) and Baharvand et al. (20). Rodrigo-Saez et al. (16) suggested that most associated diseases in adults are of autoimmune origin. Unlike in the infant group, where allergy was followed by anemia as the main associated disease, Vivas et al. (18) suggest that anemia is one of the associated diseases in infants and children. Anemia, hypothyroidism, and type 1 diabetes were the main associated comorbidities confirmed by the results of El Mehadji et al.’s study (21). In addition, the literature has shown that celiac disease is associated with autoimmune diseases, mainly type 1 diabetes and hypothyroidism (22, 23).
The serological tests were necessary for the diagnosis of the disease, although the current study’s results showed that serology was positive in 85.2% of celiac patients. The present study’s results are similar to the results of other studies (21, 24), recalling that even in infants (90.0%), children (80.7%), and (89.2%) adults, the tests mainly (IgA and IgG a-tTG, IgA endomysial antibodies, and IgA antigliadin antibodies) were significantly higher for children than adults, which underlines an excellent predictive value to confirm the diagnosis (25, 26). A study by Roujon et al. (24) showed that anti-endomysium and tissue transglutaminase IgA antibodies represent the most specific and sensitive markers, with the exception of IgA-deficient patients. Further search for IgG is recommended. The present study’s results are concordant with other studies' results (27) concerning the use of anti-transglutaminase assays. Nevertheless, the present study showed a significant difference in anti-transglutaminases of IgG (P < 0.001).
The histological test defined by a duodenal biopsy remains an examination of reference to confirm the diagnosis of celiac disease. That is why all the studied patients underwent a biopsy with the presence of villous atrophy of partial grade (Marsh = 3), which was dominant in all the patients. Moreover, the total grade (Marsh ≥ 3) was present in adults more frequently than in children. The aforementioned results were also reported by other studies (21, 27).
The response to the gluten-free diet is also necessary to confirm the diagnosis of celiac disease (26). Therefore, good adherence to the gluten-free diet was higher in children than in adults, with a significant difference (P = 0.001), which is consistent with another study’s results (28). Poor adherence to the gluten-free diet and/or lack of clinical and histological response favors the development of refractory sprue, as confirmed by Leffler et al. (28) and Abdulkarim et al. (29).
5.1. Conclusions
Celiac disease in the western Algeria region presents many differences between children and adults. In adults, the presentation of the disease is marked during childhood and is characterized by the presence of atypical and typical forms. Additionally, comorbidity was in the form of autoimmune diseases in adults. Adherence to the gluten-free diet has been well established in children than adults.