1. Background
Various factors, directly or indirectly, affect self-care behaviors in diabetic women, including biological, psychological, economic, social, cultural, and health factors. In pregnant women without a history of diabetes, due to physiological changes in glucose metabolism, carbohydrate intolerance is developed, which is associated with pregnancy and fetal complications, a condition called gestational diabetes mellitus (GDM). About 80% of GDM cases in normal pregnancies are related to pancreatic beta β-cell dysfunction along with chronic insulin resistance (1). Diagnosis of GDM is usually confirmed using an oral glucose tolerance test (GTT). Factors such as maternal age, ethnicity, geographical location, history of gestational diabetes, and familial history of type 2 diabetes mellitus (T2DM) can increase the risk of GDM (2). Sudden weight gain in the second trimester independently increases the risk of GDM (3). The prevalence of GDM has been reported as 7% in North America, 15.2% in the Middle East and North Africa, and 6.1% in Europe (2). The prevalence of gestational diabetes in the Middle East and North Africa is higher than in European countries (4). Also, the incidence rate in Iran is 5.4% (5). Multiple detrimental effects have been noted for GDM on the mother and fetus, the most common of which are maternal hypertension, preeclampsia, hypoglycemia, infections, and labor injuries. Also, the risk of developing T2DM after childbirth increases, and among fetal complications, macrosomia, polycythemia, hyperbilirubinemia, arrhythmia, and cardiomyopathy can be mentioned (6).
Zinc is an essential mineral and the main cell builder as well (7). Zinc plays a role in protein synthesis, cell division, nucleic acid metabolism, and ribosome biogenesis. Other functions of zinc can be referred to as the regulation of gene transcription, cellular signals, hormone secretion, and endocytosis. About 3,000 transcription factors contain zinc as a cofactor (8). Serum zinc concentration is directly related to the level of zinc’s dietary intake. Zinc deficiency can cause gestational diabetes, intrauterine growth restriction, increased risk of miscarriage, neural tube defects, and congenital heart abnormalities (9). Zinc also plays a role in insulin secretion and promotes the insulin signaling pathway, and it is a part of therapy in patients with T2DM for controlling blood glucose levels (10). Zinc can reduce inflammation and oxidative stress in pregnant women with GDM (11) by reducing the concentration of malonedialdehyde enzyme (MDA) (12). Zinc deficiency during the early life of newborns can cause growth retardation and cognitive impairment. Zinc also prevents macrosomal complications and reduces the newborn’s weight to normal levels (13). Dietary supplements containing zinc, along with other nutrients, can prevent hyperglycemia and the incidence of GDM (14). Considering the role of zinc in growth and development, deficiency of this element can favor the occurrence of pregnancy complications such as GDM and its subsequent adverse effects (15).
2. Objectives
Considering the importance of maternal health and delivering a healthy baby, this study was designed to compare serum zinc levels between diabetic and healthy pregnant women and to evaluate the relationship between zinc levels and some risk factors of GDM.
3. Methods
3.1. Participants
In this case-control study, 70 diabetic and healthy pregnant women (24 - 28 weeks of reproductive age) referred to the diabetes clinic of Bu-Ali Hospital in Zahedan during a 2-month period were enrolled. The participants were divided into two groups: 35 diabetic pregnant women and 35 healthy counterparts.
According to a previous study reporting an average serum zinc level of 84.84 mg/dL in diabetic pregnant women and 83.47 mg/dL in healthy pregnant women (16), the sample size was determined as 27152 using the formula of comparing the means of two populations. However, in practice, it was not possible to recruit this sample size due to limitations in blood sampling and coordination, as well as the process being time-consuming and costly. The sample size for Rahimi Sharbaf et al.’s study was n = 70, was divided into two groups (35 women with GDM and 35 healthy peers) (17).
3.2. Inclusion and Exclusion Criteria
Inclusion criteria were: (1) being 24-28-week pregnant based on sonography determined in the second trimester; (2) elevated oral GTT; (3) single pregnancy; and (4) Iranian citizenship; exclusion criteria were: (1) having a history of chronic disease; (2) a history of drug use; (3) suffering from metabolic disorders other than diabetes; (4) multiple pregnancies; (5) smoking and alcohol consumption; and (6) hypertension.
3.3. General Characteristics and Laboratory Measurements
For each of the pregnant women, demographic characteristics, including age, gestational age, body weight, height, blood pressure, and body mass index (BMI), were recorded. In order to determine the serum zinc level, 3 mL of venous blood was taken from all participants after obtaining consent and under the supervision of an obstetrician and gynecologist. After separating sera, a zinc assay kit (Bayerx Fars, code IRAN-BXC0462) was used to measure serum zinc level based on an enzymatic calorimetry method and using an automatic DIRUI CS-400 device.
3.4. Statistical Analysis
Statically analysis was performed in SPSS version 22, using mean, standard deviation, and range of variation (minimum and maximum) to present the data. For the analytical part, the independent t-test was used, and the association between different indices was studied by Pearson correlation. P < 0.05 was considered statistically significant.
3.5. Ethical Considerations
This project was approved by Ethics Committee at the Research Council of Zahedan University of Medical Sciences (ZAUMS) (ethical code: IR.ZAUMS.REC.1398.328).
4. Results
The means of age in the case and control groups were 25 ± 5.4 and 28 ± 5.1 years, and the lowest ages in the two groups were 16 and 18 years old, respectively. The oldest age in both groups was 35 years old (P = 0.1). The mean body weight in the case and control groups were 59.28 ± 10.38 and 61.24 ± 6.22 kg, respectively (P = 0.34). The means of height in the case and control groups were 158.8 ± 5.85 and 163.68 ± 6.23 cm, respectively (P = 0.001). The means of BMI in the two groups were calculated as 23.64 ± 3.34 and 22.86 ± 2.31 kg/m2, respectively (P = 0.26). Also, the mean values of systolic and diastolic blood pressure were 112.28 ± 7.31 and 75.42 ± 6.10 mmHg in the case group (P = 0.08) and 109.14 ± 7.81 and 75.42 ± 6.10 mmHg in the control group (P = 0.001), respectively. The means of gestational age in the case and control groups were 25.94 ± 1.37 and 25.45 ± 1.35 weeks, respectively (P = 0.14, Table 1).
| Groups | Weight (kg) | Height (cm) | Body Mass Index (kg/m2) | Blood Pressure Syst. (mmHg) | Pregnancy Week |
|---|---|---|---|---|---|
| Case | 59.28 ± 10.38 | 158.08 ± 5.58 | 23.64 ± 3.34 | 113.04 ± 7.30 | 25.94 ± 1.37 |
| Control | 61.24 ± 6.22 | 163.68 ± 6.23 | 22.86 ± 2.31 | 109.80 ± 7.82 | 25.45 ± 1.35 |
| P-value | 0.34 | 0.001 | 0.26 | 0.078 | 0.14 |
a No significant difference was observed between the two groups regarding the means of the general indicators studied except for height (P = 0.001) (data reported as mean ± SD).
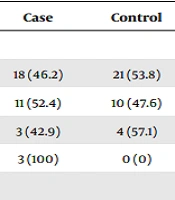
Our results showed that the mean frequencies of previous deliveries and abortion were 0.74 ± 0.95 and 0.37 ± 0.68 (P = 0.71) in women with GDM and 0.51 ± 0.7 and 0.14 ± 0.35 (P = 0.11) in healthy women, respectively (Table 2). There was no significant difference in nutritional status based on BMI (P = 0.16, Table 3) between the two groups.
| Variables | Case | Control | P-Value |
|---|---|---|---|
| Number of deliveries | 0.406 | ||
| 0 | 18 (46.2) | 21 (53.8) | |
| 1 | 11 (52.4) | 10 (47.6) | |
| 2 | 3 (42.9) | 4 (57.1) | |
| 3 | 3 (100) | 0 (0) | |
| Number of abortions | 0.188 | ||
| 0 | 26 (46.4) | 30 (53.6) | |
| 1 | 5 (50.0) | 5 (50.0) | |
| 2 | 4 (100.0) | 0 (0) |
a Values are expressed as No. (%).
b There was no significant difference between the two groups (P > 0.05). Statistical analysis was performed based on the number of previous deliveries and abortions in the case and control groups. Because 4 cells (50%) and 2 cells (33.3%) in the two groups had expected counts less than 5, respectively, Fisher’s Exact test was used.
| Study Groups | Groups, BMI (kg/m2) | Total | |||
|---|---|---|---|---|---|
| < 18 Under Weight | 18 - 25 Normal | 25 - 30 Over Weight | 30 - 35 Obese | ||
| Case | 2 (5.7) | 22 (62.9) | 10 (28.6) | 1 (2.9) | 35 (100) |
| Control | 0 (0) | 29 (82.9) | 6 (17.1) | 0 (0) | 35 (100) |
| Total | 2 (2.9) | 51 (72.9) | 16 (22.9) | 1 (1.4) | 70 (100) |
a Values are expressed as No. (%).
b χ2 = 1.94, df = 1, P = 0.16.
c Most subjects had normal BMIs. There was no significant difference between the two groups regarding the body mass index (P = 0.16).
The means of serum zinc level in the case and control groups were determined to be 62.22 ± 5.57 and 89.17 ± 12.16 μg/dL, respectively (P = 0.001, Table 4). Regarding the frequency of zinc deficiency (< 70 μg/dL), a significant difference was observed between the two groups (P = 0.001, Table 5), and there was also a significant difference between them in terms of mean fasting blood sugar (FBS), as well as 1-hour and 2-hour plasma glucose level (P = 0.001, data not shown). We observed a significant positive correlation between serum zinc level and gestational age in weeks (P = 0.03) and diastolic blood pressure (P = 0.02) in pregnant women with GDM.
| Groups, Serum Zinc Level (mg/dL) | Mean ± SD | Domaine | P-Value | |
|---|---|---|---|---|
| Min | Max | |||
| Case | 62.22 ± 5.57 | 53.00 | 76.00 | 0.001 |
| Control | 89.17 ± 12.16 | 62.00 | 110.00 | |
a There was a significant difference between the two groups regarding serum zinc level (P = 0.001). However, the range of variations in serum zinc level was lower in the case group than in the control group.
| Groups | Zinc < 70 mg/dL | Zinc ≥ 70 mg/dL | Total |
|---|---|---|---|
| Case | 32 (91.4) | 3 (8.6) | 35 (100) |
| Control | 3 (8.6) | 32 (91.4) | 35 (100) |
| Total | 35 (50) | 35 (50) | 70 (100) |
a Values are expressed as No. (%).
b χ2 = 48.5, df = 1, P < 0.001.
c There was a significant difference between the two groups (P < 0.001). The means of fasting, 1-hour, and 2-hour blood sugar levels in diabetic and healthy pregnant women were 94.8 ± 21.3 vs. 79.8 ± 7.8 (P = 0.001), 193.3 ± 38.6 vs. 146 ± 18.5 (P = 0.001), and 161.2 ± 25.6 vs. 118.2 ± 17.9 (P = 0.001), respectively.
5. Discussion
The results showed that the mean serum zinc level in pregnant diabetic women was much lower than in their healthy counterparts. In addition, zinc deficiency (i.e., serum zinc level < 70 μg/dL) in the control group (89.17 μg/dL) was higher than in the case group (62.22 μg/dL). Feng et al. reported serum zinc levels of 43.9 ± 14.5 and 62.5 ± 19.0 mg/L in pregnant women with GDM and controls, respectively (P < 0.001). Also, in this study, there was a significant relationship between serum zinc levels and indices such as gestational age (P < 0.03), diastolic blood pressure (P < 0.001), height, and insulin resistance (P < 0.001), which was in line with the findings of another study (18). In Mishu et al.’s study, serum zinc levels were significantly lower in GDM women compared to healthy pregnant women, who had normal glucose levels in the second and third trimesters of pregnancy. It is recommended to monitor serum levels of zinc and magnesium during pregnancy to prevent GDM (19). Mokhlesi et al. reported that the mean serum zinc level in women with GDM was not significantly different compared to healthy pregnant women (20). This contradictory finding compared to our observation can be due to reasons such as blood sampling in different pregnancy weeks, variety of diets, possible use of supplementations, socioeconomic conditions, and different sample sizes (20). Contrary to the results of the present study, which showed no correlation between BMI and serum zinc level in pregnant women with GDM, Rahimi Sharbaf et al. found a direct relationship between serum zinc level and BMI (17). In another study, age, BMI, and gestational age (in weeks) did not show a statistically significant difference between diabetic and healthy pregnant women, which was similar to the results of the present study (21). Wang et al. showed that the mean level of serum zinc in pregnant women with BMIs less than 18 kg/m2 was lower than in peers with normal BMIs (15). Variabilities in the findings of these studies can be due to different factors, including different cut-off points used to define zinc deficiency, age differences between the studied populations, as well as variations in economic status, gestational age, and the sample size (18).
Our results showed no significant difference between diabetic and healthy pregnant women in terms of age, the number of deliveries, and the number of abortions. Several factors seem to affect the incidence of GDM, including the low income of families in developing countries as one of the most important factors affecting zinc nutritional intake. Also, other risk factors include older age of onset of menstruation, ethnicity, family history of T2DM, obesity, history of multiple pregnancies, genetic factors, history of polycystic ovary syndrome, smoking, psychological problems, unhealthy diets, and lack of physical activity (22-24).
Evaluation of plasma zinc levels in pregnant women with gestational hypertension who had insufficient zinc intake showed that there was a relationship between maternal dietary intake of zinc, the newborn’s birth weight, and the development of severe preeclampsia syndrome with symptoms such as edema, hypertension, and proteinuria (16). There is little evidence that can show a link between zinc and spontaneous preterm birth or GDM. Studies on the link between zinc deficiency and pregnancy complications require expansion, especially in developing countries where the population is at increased risk of zinc deficiency (24). The results of this study indicated that there was a significant difference between the two groups regarding fasting, 1-hour, and 2-hour blood sugar levels, which was in line with the findings of Genova et al. (21). It seems that hypoglycemia during pregnancy in women with GDM not only decreases serum zinc levels but also can influence the amount of zinc in erythrocytes and insulin resistance. However, there is no evidence ruling out the relationship between erythrocytes’ zinc levels and insulin resistance and other glucose metabolic parameters (21). Also, the intolerance of carbohydrates during pregnancy can increase the incidence of gestational diabetes. On the other hand, other studies have shown that fasting blood sugar can be improved by receiving zinc supplementation secondary to the activation of pancreatic beta-cells, which are involved in insulin resistance and insulin sensitivity in pre-diabetes patients. These data show that zinc is an essential element for the functionality of beta cells, the crystallization of insulin as a hexamer, and the regulation of beta cells’ function by inducing insulin and antioxidant factors (25).
In addition, zinc homeostasis during pregnancy is partly regulated by placental hormones, and failure in pregnancy can affect serum zinc levels and lead to undesirable outcomes (26). Reduced serum zinc levels in diabetic women can be due to changes in the transfer of biochemical compounds to the fetus, leading to impaired gene expression (13). The present study showed a significant reduction in zinc levels in pregnant women with GDM compared to their healthy peers. However, there was a correlation between serum zinc level, gestational age in weeks, and diastolic blood pressure. Another study, however, concluded no relationship between serum zinc level and GDM (20). On the contrary, Rahimi Sharbaf et al. found a significant relationship between reduced serum zinc levels and the development of GDM (17). In the present study, blood sampling was performed in the 24 - 28 weeks of gestation, but in the studies of Mokhlesi et al. (20) and Rahimi Sharbaf et al. (17), blood sampling was performed in the 14 - 20 weeks and 24 - 28 weeks of gestation, respectively. It seems that with the progression of pregnancy, serum zinc levels decrease physiologically in parallel with increasing fetal needs (20).
5.1. Conclusions
The results of the present study showed that most pregnant women with GDM had severe zinc deficiency, which can exacerbate the complications of GDM. However, zinc deficiency should be studied in relation to other demographic, racial, nutritional, socioeconomic, and ecological factors, which requires more extensive research and continuous and accurate monitoring. In the present study, indicators such as gestational age and diastolic blood pressure were associated with zinc deficiency. As pregnancy progresses and the needs of the fetus increase, serum zinc decreases, altering insulin metabolism and function. It seems that screening for zinc deficiency during pregnancy, in parallel with other diagnostic tests and therapeutic methods, can prevent pregnancy complications and improve the quality of pregnancy. Also, the possibility of using zinc supplements as a therapeutic strategy during pregnancy requires more extensive studies.