1. Background
Sleep and circadian systems are tightly intertwined. In most cases, both work together to adapt the organism to the constant change of daily needs; otherwise, the body will not function properly. Therefore, during the sleep-wake cycle, there are significant changes not only in physical and mental activity, cardiovascular function, and temperature regulation but also in immune parameters, such as the number, function, and proliferation of leukocytes and the production of cytokines (1). Sleep homeostasis disorder is usually accompanied by the increased activity of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in increased levels of stress hormones, such as cortisol, which is one of the main hormones that suppress the immune system (2). The activation of the immune system has been reported to interfere with sleep, and on the other hand, sleep affects the innate and acquired parts of the body’s defense system; therefore, adequate night sleep has been suggested as a treatment for infectious diseases (3).
Research findings show that in healthy subjects, the antibody response to hepatitis A (4) and influenza (5) vaccines in sleep-deprived individuals is significantly lower than in those with normal sleep. Another study reported that healthy subjects with an average of less than 7 hours of night sleep had approximately 3 times less cold than those with 8 hours or more of sleep (6). Additionally, continuous sleep deprivation has been associated with hypercatabolic status, increased metabolism, increased plasma levels of norepinephrine, symptoms of malnutrition, and increased mortality in rats (7).
There is conflicting evidence of the effects of sleep deprivation on immune system parameters and hormones that affect them. Some reports indicate an increase in leukocyte counts, immunoglobulin A (IgA), and cortisol (8-10); however, there are other studies that show that sleep deprivation has no effect on these parameters (11, 12). On the other hand, in other studies, changes in immune responses to acute exercise following sleep deprivation have been reported (10, 13, 14); however, the effects of long-term exercise on immune and hormonal parameters after sleep deprivation have not been investigated, and to the best of our knowledge, there is no study in this area. Considering the role of sleep deprivation and subsequent drowsiness on occupational accidents and the spread of physical and mental diseases, such as obesity, type 2 diabetes, and depression (5, 15), understanding the effects of aerobic training on immune and hormonal responses to sleep deprivation can pave the way for developing appropriate strategies to prevent these events and reduce complications.
Despite the fact that everyone is aware of the importance of getting enough sleep, sleep deprivation is still a common phenomenon in today’s society, especially among the younger generation. In Iranian society, most young individuals, after returning from work or university, are busy with things such as watching TV and going to the cinema. Sleeplessness in Iran has become a problem among young individuals (16). Additionally, sometimes, some individuals, such as nurses, drivers, military personnel, pilots, and firefighters, experience nighttime sleeplessness due to occupying certain occupations. On the other hand, immune system disorders due to sleeplessness and sleep deprivation have become largely apparent. Therefore, developing appropriate medical strategies to deal with the complications and consequences of sleeplessness and sleep deprivation is essential.
Based on the available reports, there is limited consistent information about the effects of sleep deprivation on immune and hormonal parameters. In addition, based on searching, no study has investigated the effects of long-term exercise on the changes of these parameters following sleep deprivation. On the other hand, during the onset of coronavirus disease 2019 (COVID-19), maintaining the function of the immune system to fight against the disease is essential.
2. Objectives
Since exercise and sleep deprivation affect immune function, the present study was conducted to investigate the effect of 8 weeks of aerobic training on the response of immune and hormonal factors to 30 hours of sleep deprivation in young women.
3. Methods
In this semi-experimental study, 21 young volunteer women within the age range of 20 - 40 years were recruited. After explaining the benefits and risks, the participants were randomly divided into two control (n = 10) and aerobic training (n = 11) groups. The subjects’ inclusion criteria comprised no history of sleep disorders, being healthy, no smoking, and no use of supplements, alcohol, caffeinated substances, and medication. Moreover, the subjects had normal sleep during the week before the study and did not travel across time zones.
Based on the information obtained from the medical questionnaire, individuals with a history of cardiovascular, hepatic, renal, and pulmonary diseases, diabetes, irregular menstrual cycle, drug use, and smoking, and subjects with participation in less than 80% of training sessions were excluded from the study process.
Initially, a session was held to familiarize the subjects with the research process, benefits, and possible risks. All subjects completed an informed consent form for medical history and readiness to begin physical activity. Then, anthropometric (i.e., height, weight, and body mass index (BMI)) and physiological (i.e., maximum oxygen consumption (VO2 max)) indices were measured. The Rockport one-mile walk test was used to measure VO2 max (16). Afterward, based on the schedule, the protocol was implemented in three separate stages as follows:
3.1. Stage One (Pre-test)
This stage consisted of 30 hours of sleep deprivation in which all participants were deprived of sleep from 07:00 a.m. until 13:00 p.m. on the following day. Then, the blood samples were taken from the subjects. Legitimate entertainment, such as computer games, reading books, and watching movies and TV series, was used to keep participants awake during sleep deprivation. During this period, the participants did not use any caffeinated substances to tolerate sleeplessness. The sleep deprivation protocol used in the present study was adapted from another study (16).
3.2. Stage Two (Undergoing Aerobic Training)
Following 2 days, the subjects in the experimental group followed an aerobic training protocol, including 30 - 50 minutes of continuous running 3 days a week for 8 weeks with an intensity of 55 - 65% of heart rate reserve (HRR). However, the subjects in the control group continued their lives in a sedentary way. Each aerobic training session for the experimental group started with 5 minutes of warm-up, then continued with a special program for each session, and ended with 5 minutes of cool-down. All training programs were performed under the supervision of the researcher.
3.3. Stage Three (Post-test)
This stage included 30 hours of sleeplessness in which the subjects of both groups were deprived of sleep at least 48 hours after the last training session from 07:00 a.m. to 13:00 p.m. on the following day. Then, the blood samples were taken from the subjects, and then anthropometric and physiological indices were measured.
Using VENOJECT needles, the blood samples were collected at each stage at the rate of 5 cc from the brachial vein of the subjects in a sitting position to measure biochemical variables. Then, the blood samples were immediately poured into tubes containing K3-EDTA anticoagulant and were centrifuged for 18 minutes at 1500 rpm, and after serum separation, they were poured into 0.5 cc microtubules.
Serum IgA and immunoglobulin G (IgG) concentrations by the single radial immunodiffusion (SRID) method using special plates (Baharafshan model, Iran), serum cortisol concentrations by enzyme-linked immunosorbent assay (ELISA) (DiaMetra model, Italy), and blood leukocyte counts using a cell counter model (Sysmex K21N, Japan) were measured.
The data were reported based on mean ± standard deviation. Using the Shapiro-Wilk test, the normality of data distribution was assessed. The mean values of the variables before and after the intervention in both groups were compared using the paired t-test. In addition, an independent t-test was used for the comparison between the two groups. All data were analyzed using SPSS software (version 22) at a significance level of P < 0.05.
4. Results
Table 1 shows the anthropometric and physiological characteristics of the subjects in both groups before and after the intervention. There was no significant difference in weight and BMI between the two groups in the pre-test and post-test (P > 0.05). Nevertheless, after the intervention, VO2 max in the aerobic training group showed a significant increase compared to the control group (P = 0.001).
| Variables | Groups | Between-Group Differences (P-Value) | |
|---|---|---|---|
| Control (n = 10) | Aerobic Training (n = 11) | ||
| Weight (kg) | 0.092 | ||
| Pre-intervention | 59.8 ± 5.2 | 57.4 ± 4.3 | |
| Post-intervention | 59.4 ± 5 | 55.5 ± 3.7 | |
| Within-group differences (P-value) | 0.924 | 0.063 | |
| BMI (kg/m2) | 0.157 | ||
| Pre-intervention | 23.92 ± 2.7 | 23 ± 2.2 | |
| Post-intervention | 23.76 ± 2.6 | 22.2 ± 2 | |
| Within-group differences (P-value) | 0.981 | 0.104 | |
| VO2 max (mL/kg/min) | 0.001 | ||
| Pre-intervention | 34.8 ± 4.3 | 35.2 ± 4.6 | |
| Post-intervention | 35.3 ± 4.5 | 44.7 ± 5.2 b | |
| Within-group differences (P-value) | 0.897 | 0.0001 | |
Abbreviations: BMI, body mass index; VO2 max, maximum oxygen consumption.
a The data are expressed as mean ± standard deviation.
b Significant increase rather than in the control group.
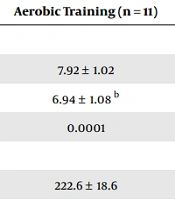
As shown in Table 2, after the intervention, leukocyte counts (P = 0.0001), serum IgA concentrations (P = 0.0001), and cortisol (P = 0.023) levels in response to 30 hours of sleep deprivation significantly reduced in the aerobic training group. However, serum IgG concentrations (P = 0.084) did not show a statistically significant change in response to 30 hours of sleep deprivation in the 2 groups of aerobic training and control (Table 2).
| Variables | Groups | Between-Group Differences (P-Value) | |
|---|---|---|---|
| Control (n = 10) | Aerobic Training (n = 11) | ||
| Leukocyte counts (103 cells/μL) | 0.0001 | ||
| Pre-intervention | 7.60 ± 1.00 | 7.92 ± 1.02 | |
| Post-intervention | 7.75 ± 1.03 | 6.94 ± 1.08 b | |
| Within-group differences (P-value) | 0.935 | 0.0001 | |
| Serum IgA (mg/dL) | 0.0001 | ||
| Pre-intervention | 215.7 ± 19.3 | 222.6 ± 18.6 | |
| Post-intervention | 219.8 ± 19.7 | 209.3 ± 13.9 b | |
| Within-group differences (P-value) | 0.966 | 0.0001 | |
| Serum IgG (mg/dL) | 0.084 | ||
| Pre-intervention | 1130.7 ± 183.3 | 1231.1 ± 253.8 | |
| Post-intervention | 1176.2 ± 162.5 | 1107.6 ± 206.1 | |
| Within-group differences (P-value) | 0.902 | 0.054 | |
| Serum cortisol (ng/mL) | 0.023 | ||
| Pre-intervention | 152.1 ± 26.5 | 146.2 ± 24.8 | |
| Post-intervention | 149.5 ± 22.7 | 131.9 ± 21.4 b | |
| Within-group differences (P-value) | 0.879 | 0.001 | |
Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G.
a The data are expressed as mean ± standard deviation.
b Significant decrease rather than in the control group.
5. Discussion
Based on the results of the present study, it was found that 8 weeks of aerobic training significantly reduced the leukocyte counts, serum IgA, and cortisol concentrations in response to 30 hours of sleep deprivation in young women, although IgG concentrations did not show any change. The evidence suggests that reductions in leukocyte counts are achieved at or above levels of aerobic training recommended for general health. Training combined with interventions that specifically affect abdominal obesity and the concentrations of interleukin 6 (IL-6) and adiponectin are more effective in reducing leukocyte counts (17). Based on our knowledge, the present study is the only study that examined the response of immune cells to 1 night of sleep deprivation. Therefore, to expand the results, this study is compared to studies that have investigated the effects of acute sleep deprivation on immune parameters.
The findings of the present study on the effect of aerobic training on reducing leukocyte counts following 30 hours of sleep deprivation in young women are in conflict with the results of other studies (10, 11, 18). An increase in leukocyte, lymphocyte, and monocyte counts after a period of 5 nights of sleep deprivation (4 hours of night sleep) has been reported (10). Although in the present study, sleep deprivation for 30 hours might have increased these indicators, 8 weeks of aerobic training reduced the counts. The effect of 29 hours of sleep deprivation was investigated using healthy human subjects in another study, and the results showed that leukocyte counts did not change (11). However, in the present study, in addition to sleep deprivation, the subjects underwent aerobic training for 8 weeks. Moreover, contrary to the findings of the present study, it was reported that after 2 nights of sleep deprivation in healthy subjects, leukocyte counts increased compared to baseline levels (18). The main reason for the difference between the results of the above-mentioned study (18) and the present study is the lack of exercise in the above-mentioned study because no exercise intervention was used in that study.
There are several mechanisms to explain the decrease in leukocyte counts following exercise. Exercise might alter the exchange of leukocyte subtypes between secondary lymphatic organs and the blood. In addition, exercise might directly affect bone marrow hematopoiesis and therefore causes changes in leukocyte counts in the blood. The growing evidence suggests that the activation of innate and acquired immune cell subtypes increases inflammation during obesity (19). Decreased obesity and restoration of adipokine balance (i.e., decreased leptin and increased adiponectin) associated with exercise might be involved in changes in leukocyte exchange, hematopoiesis, or leukocyte circulation (20). Obesity is strongly associated with increased circulating leptin concentrations, and leptin has been shown to activate neutrophils and induce the production of proinflammatory cytokines (e.g., IL-6 and tumor necrosis factor alpha (TNF-α)) (21, 22). Long-term exercise has been shown to reduce IL-6 and TNF-α levels, resulting in a decrease in the circulating leukocytes and neutrophils counts (23).
Dixon and O'Brien reported that total leukocyte count was associated with BMI, and decreasing it through weight loss was associated with an 11.7% decrease in neutrophils and a 6.9% decrease in lymphocytes (24). In the present study, leukocyte count decreased by 12.3% after aerobic training, which was probably due to a decrease in neutrophil and lymphocyte counts. Furthermore, leukocyte count might be affected by factors that regulate the body’s hormonal responses to exercise. In particular, the activation of the HPA axis and the release of corticosteroids (cortisol) might play a major role in how immune cells are distributed after exercise (25). Therefore, it is possible that in the current study, performing 8 weeks of aerobic training by modulating adipokine balance, reducing corticosteroids (cortisol), proinflammatory cytokines, and BMI decreased the leukocyte count at rest and declined its number after 30 hours of sleep deprivation.
Another finding of the current study was that 8 weeks of aerobic training significantly reduced serum IgA levels in response to 30 hours of sleep deprivation in young women; however, it had no effect on serum IgG levels. A review of the related literature shows that no study has been conducted on the effect of long-term training on the response of serum immunoglobulins to sleep deprivation. However, a few studies have examined the effect of sleep deprivation on immunoglobulins levels in response to aerobic activity. Consistent with the findings of the present study, it has been reported that 30 minutes of aerobic activity on an ergometer cycle with an intensity of 70 - 75% of maximum heart rate has no effect on serum IgG concentrations following sleep deprivation in young men (26). One reason for the consistency of the results is the similarity of sleep deprivation and the type of training between the studies. In contrast, 30 hours of sleep deprivation increased salivary IgA concentrations in response to submaximal exercise (27).
Changes in serum immunoglobulin levels due to exercise training are due to several factors because the regulation of antibodies is a complex phenomenon and involves different types of cells, including B cells and T-messenger molecules (cytokines) (28). Performing high-intensity exercise changes the ratio of lymphoid cells in the bloodstream and lymphoid tissues, resulting in an increase, decrease, or no alteration in the levels of serum antibodies (25). Furthermore, performing high-intensity exercise is associated with changes in lymph flow and the release of various proteins into the bloodstream, which might bring about changes in the levels of serum antibodies (28). Therefore, it is likely that following aerobic training in the study, the ratio of lymphoid cells and lymph flow changed, weakening the serum IgA response to a sleepless night. Hormonal changes due to exercise are other factors affecting the levels of serum immunoglobulins. It has been shown that increases in hormones, including cortisol and catecholamines, following strenuous exercise are likely to alter serum immunoglobulins levels (29).
The increased secretion of cortisol and other stress hormones, such as epinephrine, reduces the function of the immune system and the proliferation of lymphocytes (25); therefore, a decrease in serum cortisol concentrations (as it occurred as a result of aerobic training in the present study) might increase B lymphocyte proliferation and consequently increase serum immunoglobulins levels. In this context, corticosteroids reduce the circulating lymphocyte counts by blocking the entry of lymphocytes into the bloodstream and facilitating their exit, thereby reducing immunoglobulins (28). Therefore, it is possible that in the current study, due to decreased cortisol and altered HPA activity, an increase in immunoglobulins occurred after aerobic training. As a result, immune system adaptation is achieved, and there is a smaller increase in serum IgA in response to sleep deprivation.
In this study, after 8 weeks of aerobic training, serum cortisol concentrations were significantly reduced after 30 hours of sleep deprivation in young women. A review of the related literature shows that limited attention has been paid to the effects of regular exercise on hormonal responses to sleep deprivation. Most of the previous studies have examined the acute effects of exercise on hormone levels following sleep deprivation. For example, in contrast to the results of the present study, it was reported that submaximal exercise increased serum cortisol concentrations (27). One reason for the conflict between the results is the intensity of exercise and the study protocol because the researchers in their study used a session of submaximal exercise after 1 night of sleep deprivation. Additionally, in contrast to the findings of the present study, serum cortisol concentrations were significantly increased in male athletes following sleep deprivation and high-intensity anaerobic exercise (13). The reasons for the discrepancy in results might include the mode of exercise performed (i.e., high-intensity anaerobic versus moderate-intensity) and the gender of the subjects.
A variety of causes for changes in cortisol concentrations, as a stress hormone, has been suggested, including HPA stimulation, adrenocorticotropic hormone (ACTH) secretion, central body temperature changes, pH changes, sympathetic nervous system stimulation, and psychological stress (30, 31). In addition, decreased resting cortisol levels after long-term exercise have been attributed to increased circulating cortisol removal and decreased ACTH activity (32). It seems that by reducing the stimulants of cortisol production (e.g., ACTH and psychological stress) following the aerobic training in the present study, its resting levels are reduced, and as a result, the effect of 30 hours of sleep deprivation is attenuated.
5.1. Conclusions
Overall, the results of the present study showed that 8 weeks of moderate-intensity aerobic training attenuated the disruption of immune and hormonal responses to 30 hours of sleep deprivation in young women. Aerobic training can modulate the negative effects of short-term sleep deprivation on the immune system. Nonetheless, further research is needed to make a definitive statement in this regard.