1. Background
COVID-19 disease was declared a global pandemic on March 11th, 2020, by the World Health Organization (WHO) (1). Preventive strategies and an active lifestyle are two important factors that decrease the risk of contracting COVID-19 (2). Prevention strategies are widely implemented worldwide, including personal protective activities, social distancing, and environmental cleaning. Governments closed many public places and sports clubs and canceled sports events to protect people from the disease. With the closure of sports clubs and gyms, most bodybuilders suffered from under-training or a total lack of training. Meanwhile, athletes infected with COVID-19 had to spend some time in quarantine without training. Only some studies have investigated the effect of the lack of exercise during the quarantine period (3), leaving questions concerning the time to return to training and the intensity of exercise necessary to offset the deleterious effects of the virus unanswered. It appears that athletes with minor or moderate disease, after complete recovery with seven to ten days of rest, can return to training. Cardiac and respiratory tests should more closely examine athletes requiring treatment for over 14 days before returning to exercise to minimize the risk of virus-induced myocardial infarction and thromboembolic incidents (3).
Consequences that may follow the lack of training in quarantine include anthropometric and physiological changes. The reversibility principle states that when regular exercise activity is significantly reduced or stopped, it causes a partial or complete reduction of anatomical, physiological, and functional adaptations depending on the duration of lack of training (4, 5). Therefore, determining the intensity of training for returning to exercise is important, especially for strength training athletes who usually exercise with nearly maximum loads or to muscular fatigue. Several studies have demonstrated that excessive physical activity can impair immune function, inflammation, oxidative stress, and cause muscle damage (6, 7). Inflammatory cytokines alter immune function following strenuous and long-term exercise (8, 9). This is more prominent in resistance training athletes. Neutrophil and NK cell functions, cytokines, the expression of major histocompatibility complex type II in macrophages, and markers of immune function are reduced from a few hours to several days after long-term and intense endurance sports activities (10). However, in untrained individuals, more severe responses in the immune system parameters can ensue (11). Similarly, for athletes unable to exercise continuously for some time due to illness, starting exercise is crucial in returning to functional capacity with no injury. Immune-specific proteins (lysozyme C, neutrophil elastase, defensin-1, the antimicrobial peptide cathelicidin) are produced to regulate the innate immune response (chemotoxic and translocation), and oxylipins are involved in initiating, mediating, and resolving this process. Other proteins, such as amyloid A4, myeloperoxidase, and complements, increase during the recovery phase and act in response to the acute inflammatory phase (12). These disturbances in metabolism, lipid mediators, and proteins induced by exercise directly affect immune functions, decrease immune cells' capacity and increase oxygen consumption after activation. Primary data showed that the metabolic capacity of immune cells decreases during recovery from periods of intense activity, which leads to transient immune dysfunction (13). However, more research is required to draw a definitive conclusion. On the other hand, it should be noted that Covid-19 disease is still prevalent worldwide, and it seems that even if the COVID-19 disease pandemic is controlled, it will remain a seasonal disease. Therefore, investigation of clinical manifestations during exercise and anthropometric and physiological changes in bodybuilders after COVID-19 can familiarize sports trainers with the condition of those who recover from the disease and provide them with an appropriate model for regulating exercise programs. It seems that no study has examined this issue in any sport hitherto.
2. Objectives
The purpose of the current study was to measure and compare selected anthropometric and physiological parameters in bodybuilders who experienced different training restrictions due to COVID-19.
3. Methods
The current study was of quasi-experimental pre-test and post-test type. The basic procedures of this study are shown in Table 1.
| Place of Practice and Test | Turqan Sports Complex |
|---|---|
| Time of exposure to COVID | May to July |
| Time to return to training athletes without training | August |
| Time to return to exercise in coronary patients | May-October |
| Exercise completion time | October |
| Data collection | Before the disease every month and the final test in October |
| Time of contracting COVID-19 | July to August |
| Duration without practice | August to September |
| Temperature | 21 - 23 degrees |
| Humidity | 40 - 50 percent |
| Sports activities | They had no exercise other than the training protocol |
The research sample size of 36 participants for each group was computed using G*power software version 3.1.2 for the relevant statistical tests with a statistical power of 0.95, an effect size of 0.70, and an alpha level of 0.05 (14). Bodybuilders from northwestern Iran (age = 24 - 33 years) with two years of training experience volunteered to participate. All participants were in good physical and mental health and had been training voluntarily for at least six months under the supervision of the team's specialists before the pandemic. All had performed resistance training regularly with no injuries that prevented them from participating. All participants consumed two grams of protein per kilogram of body weight daily, with 50 - 55% of their diet comprising carbohydrates. They did not have other daily training activities and had ample sleep during the night.
3.1. Procedures
All athletes exercised under the supervision of a coach. Body composition and physiological profiles were examined monthly before the occurrence of the disease. Twelve athletes who continued training during the pandemic were selected as a continuation group (CTR). Twelve athletes (HWT) who could not continue their training due to the closure of clubs and remained without training for six weeks were selected as the second group. The third group (INF) comprised 12 athletes who contracted mild-to-moderate COVID-19 during the pandemic and were without training for six weeks. The symptoms of infected athletes were fever, headache, cough, anorexia, joint pain, lethargy, sore throat, fatigue, and dizziness, and only three athletes had lung involvement. Their PCR test was negative after 15 days and about three weeks after their infection; also, despite their infection with the virus, they had no cardiac or respiratory symptoms at the time of the study. Therefore, these athletes did not exercise for six weeks. In the assessment before their return to sports activities, according to previous recommendations (3, 4), cardiovascular symptoms such as chest pain, palpitations, dizziness, syncope, tachycardia, and respiratory symptoms such as cough, sneezing, sore throat, asthma, and post-viral bronchial hyperactivity were examined. Participants were asked to perform on an elliptical trainer for 10 minutes. If the condition of the participants did not change and they had no muscle pain, fever, or gastrointestinal symptoms, they could engage in light to moderate exercise and gradually return to full physical activity (3). All athletes volunteered to participate and signed informed consent. Because the participants were used to train, some of their anthropometric and physiological parameters were measured and recorded at their club on a monthly basis, and these records were used as the pre-test values. Four weeks after recovery and a negative PCR test, the post-test values for all parameters were measured.
3.2. Instruments
In both pre and post-training stages, skinfolds were measured using Slim Guide calipers according to the International Society for the Advancement of Kinanthropometry criteria. All measurements were performed between 17:00 and 19:00. Each skinfold was measured twice; if readings were more than one millimeter different, they were measured a third time. Lean body mass (LBM) was calculated as body weight (weight × %fat/100). Athletes were advised not to consume heavy meals for three hours before the test, and they had free access to water while training.
To estimate maximal muscular strength, participants performed the chest press test for the upper body and the squat test for the lower body (15). Strength tests were performed with the support of two spotters. The athletes were instructed to attempt at least two repetitions for maximum measurement. If the athlete was successful, a five-minute rest was given, weights were added, and another attempt was made until they could perform only one repetition.
The training protocol was based on NASM strength and bodybuilding training warm-up and resistance training protocols, as summarized in Table 2.
| Warm-up | |||||
|---|---|---|---|---|---|
| Sport Activity | Sets | Duration | Exercise Notes | ||
| Biking | 1 | 5 minutes | Medium speed, low resistance | ||
| Active isolated stretching: Stretch the whole body | 2 | 10 times | Stretch every part for 1 to 2 seconds | ||
| Resistance Training | |||||
| Exercise | Sets | Repetition | Tempo | Rest | IRM |
| Barbell shoulder press | 3 | 12 | 2 - 1 - 2 | 90 seconds | 60% |
| Standing barbell curl | 2 | 12 | 2 - 0 - 2 | 60 seconds | 60% |
| Hack squat | 3 | 12 | 2 - 0 - 3 | 90 seconds | 60% |
| Barbell skull crushers | 3 | 12 | 2 - 0 - 2 | 70 seconds | 60% |
| Front lat pulldown | 3 | 12 | 2 - 1 - 2 | 90 seconds | 60% |
| Barbell bench press | 3 | 12 | 2 - 0 - 2 | 90 seconds | 60% |
| Lying leg curls | 3 | 12 | 2 - 1 - 2 | 90 seconds | 60% |
| Dumbbell shrugs | 3 | 12 | 2 - 1 - 2 | 70 seconds | 60% |
| Plank | 3 | 45 seconds | - | 70 seconds | Body weight |
| Sport activity | Sets | Duration | Exercising notes | ||
| Biking | 1 | 3 minutes | Low speed, no resistance | ||
| Static stretching: All active muscles | 2 | 10 seconds | Stretch each part for 10 seconds | ||
3.3. Statistics
The Shapiro-Wilk test was used to assess the normality of the data distribution. Descriptive tests (mean and standard deviation) were used to describe data. Paired t-test was used to compare the pre-test and post-test. One-way analysis was also used to compare the groups. The Tukey post-hoc test was used for an intergroup comparison with a significance level of P < 0.05 using SPSS version 22 software. Graphs were drawn using Graph Pad Prism 9 software.
4. Results
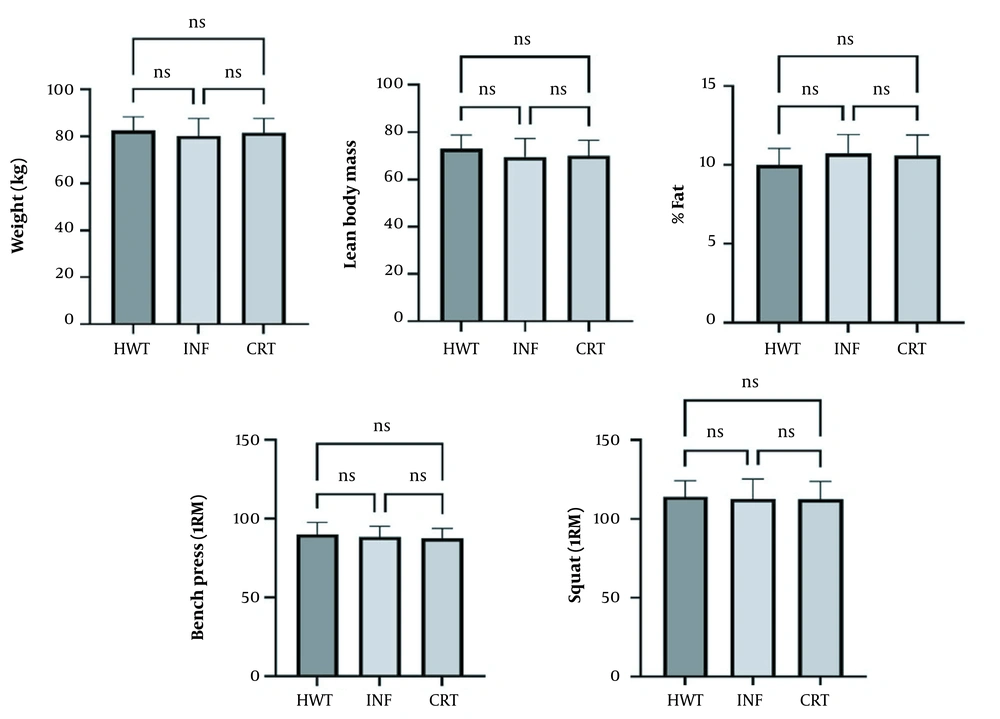
The ANOVA pre-test results showed no significant relationship between the groups (Figure 1).
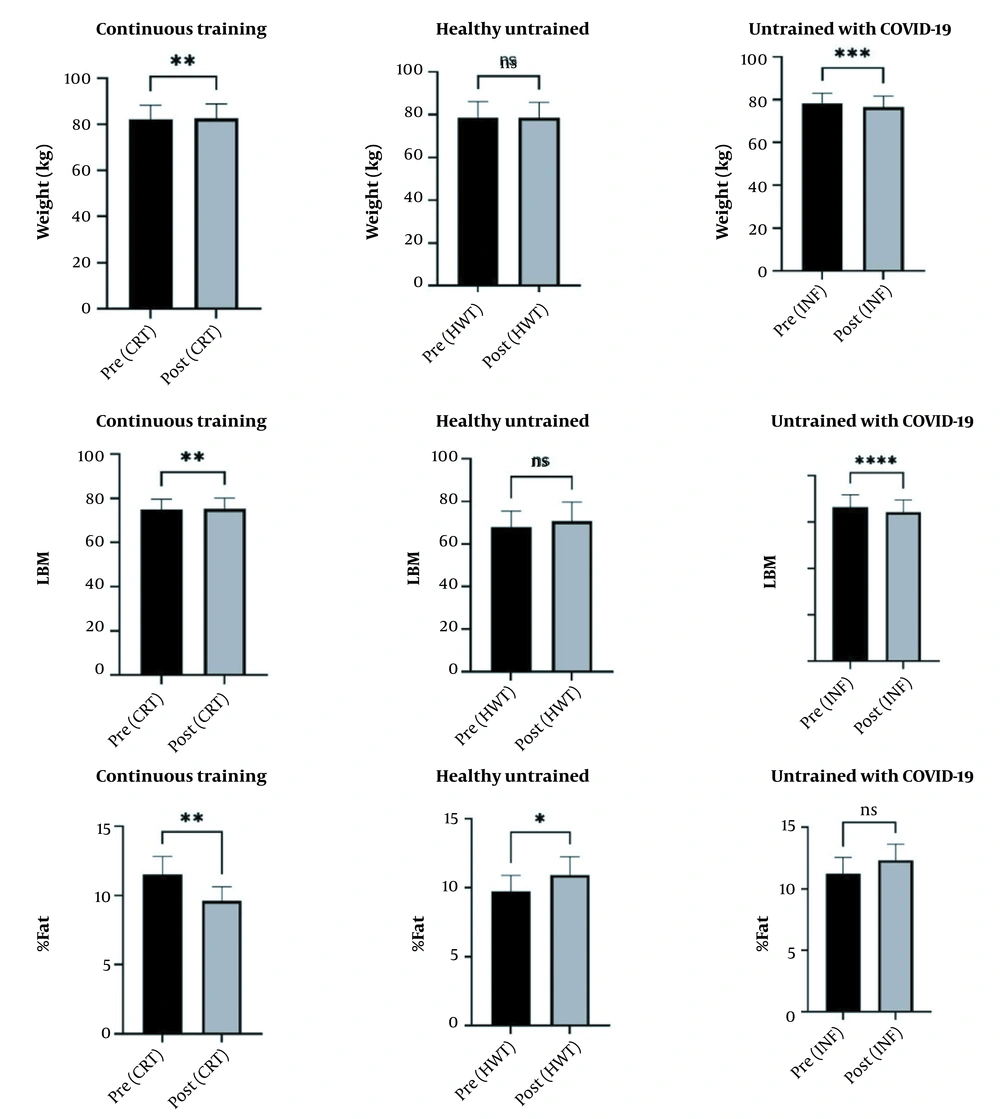
The paired t-test showed that weight and LBM index in CRT and INF athletes decreased significantly (P < 0.05). However, the fat percentage increased significantly only in the HWT group (P < 0.05) (Figure 2).
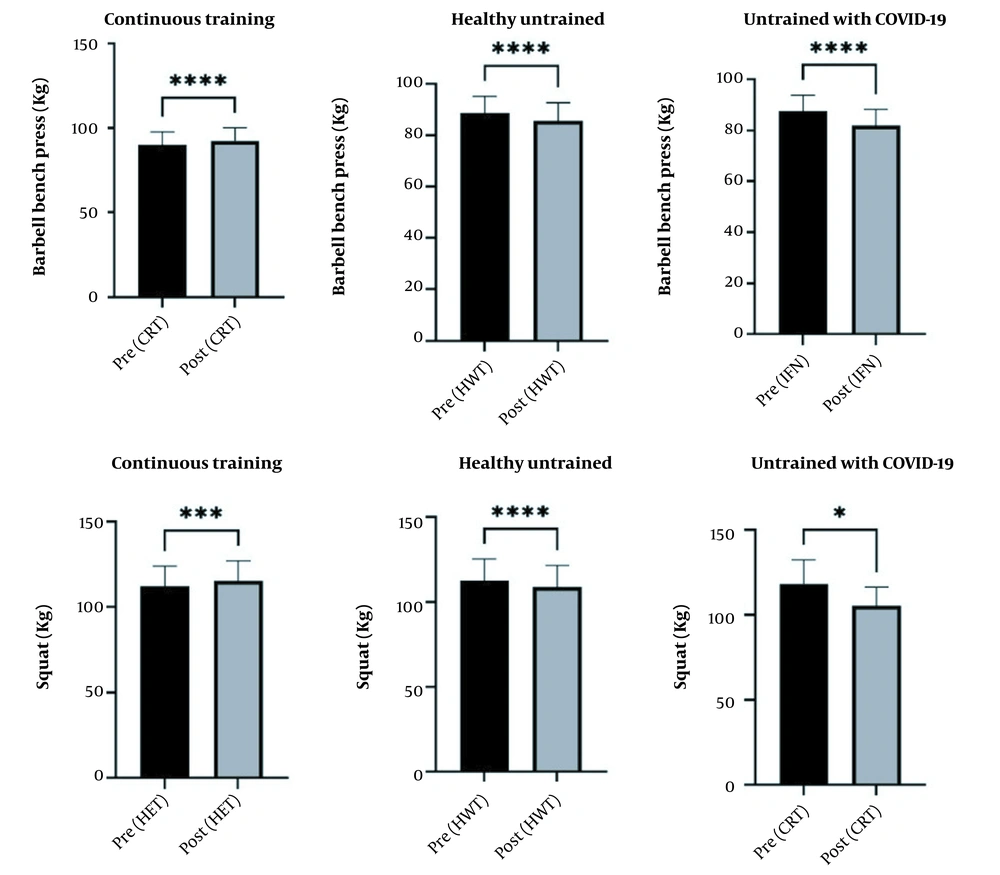
The findings also showed that the group INF had a greater decrease in one-repetition maxima in squat and chest press than the other groups (Figure 3).
The results of the ANOVA test showed that all anthropometric and physiological indices were significantly different between the three groups after HWT and INF (Table 3).
| Parameter | Groups | Mean + Standard Variation | F | Effect Size | P |
|---|---|---|---|---|---|
| Weight | HWT | 83.28 ± 6.187 | 3.992 | 0.026 | 0.028 |
| INF | 78.91 ± 7.452 | 0.027 | |||
| CRT | 76.75 ± 5.5 94 | 0.027 | |||
| LMB | HWT | 74.15 ± 6.42 | 7.336 | 0.01 | 0.004 |
| INF | 69.16 ± 8.12 | 0.011 | |||
| CRT | 66.61 ± 5.96 | 0.011 | |||
| Body fat (%) | HWT | 10.62 ± 1.01 | 12.273 | 0.014 | 0.001 |
| INF | 11.92 ± 1.28 | 0.014 | |||
| CRT | 12.89 ± 1.30 | 0.013 | |||
| Bench press 1RM | HWT | 92.41 ± 7.64 | 6.919 | 0.008 | 0.003 |
| INF | 85.66 ± 7.02 | 0.008 | |||
| CRT | 81.91 ± 6.28 | 0.008 | |||
| Squat 1RM | HWT | 117.50 ± 9.87 | 3.71 | 0.025 | 0.035 |
| INF | 108.75 ± 12.80 | 0.025 | |||
| CRT | 105.25 ± 11.08 | 0.025 |
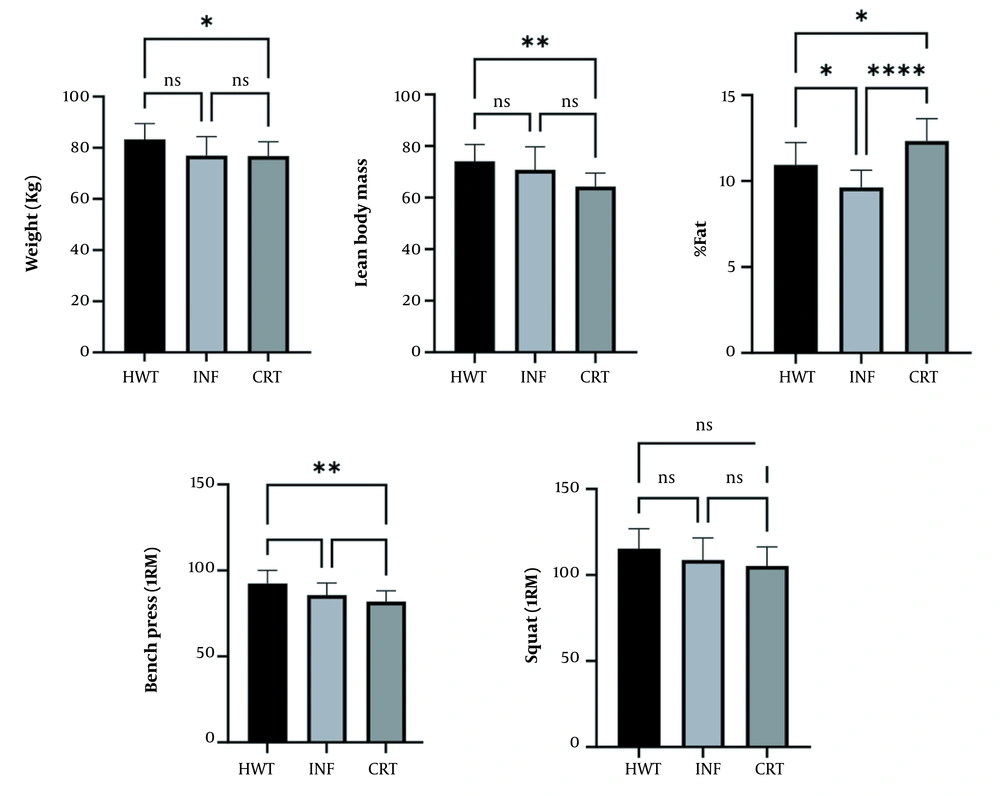
The Tukey post-hoc test results showed significant differences in weight, lean body mass, percentage of fat, and upper and lower body strength in bodybuilders (CTR) who continued their training compared to those (INF) who could relate due to COVID-19 infection. The differences between bodybuilders without training (HWT) and those who were able to continue training (CRT) were only in lean mass and fat percentage (Figure 4).
Table 4 shows that all clinical manifestations of the disease had remitted in the third week, and the recovered bodybuilders continued their training without any symptoms.
| Groups | 1st Week | 2nd Week | 3rd Week |
|---|---|---|---|
| Low blood pressure | |||
| Yes | 2 | 1 | 0 |
| No | 10 | 11 | 12 |
| Delayed soreness | |||
| Yes | 6 | 1 | 0 |
| No | 6 | 11 | 12 |
| Hypoglycemia | |||
| Yes | 2 | 0 | 0 |
| No | 10 | 12 | 12 |
| Shortness of breath during exercise | |||
| Yes | 4 | 1 | 0 |
| No | 8 | 11 | 12 |
| Joint pain | |||
| Yes | 0 | 0 | 0 |
| No | 12 | 12 | 12 |
| Coughing | |||
| Yes | 3 | 2 | 0 |
| No | 9 | 10 | 12 |
| Chest pain | |||
| Yes | 2 | 1 | 0 |
| No | 10 | 11 | 12 |
| Palpitations | |||
| Yes | 1 | 1 | 0 |
| No | 11 | 11 | 12 |
| Gastrointestinal problems | |||
| Yes | 2 | 0 | 0 |
| No | 10 | 12 | 12 |
| Syncope | |||
| Yes | 0 | 0 | 0 |
| No | 12 | 12 | 12 |
| Tachycardia | |||
| Yes | 0 | 0 | 0 |
| No | 12 | 12 | 12 |
5. Discussion
The purpose of the current study was to measure and compare selected anthropometric and physiological parameters among bodybuilders who were virus-free and continued to train (CRT) versus those who were virus-free but ceased to train (HWT) and those who were infected and ceased to train (INF). Results demonstrated that the body weights of the athletes infected with COVID-19 (INF) and those who ceased to train (HWT) were significantly reduced compared to healthy athletes who continued to exercise (CTR). This reduction was due to significant decreases in LBM of HWT and INF compared to CTR (Figure 3). Examining the components of body composition, it was noted that weight reduction was largely due to decreased LBM in HWT and INF, which did not differ significantly. This agrees with Carvalho et al. (16) findings that during three months without training, older women lost an average of three kilograms of body weight, and their functions were reduced. Loss of weight might have been due to COVID-19 infection since skeletal muscle has one or more combinations of angiotensin-converting enzymes (ACE2) and transmembrane cellular serine protease type 2 (TMRRSS2) receptors that are potential targets of the virus. Second, increased inflammatory cytokines may injure muscles (17). Another possible reason for these changes in body composition may be the side effects of treatment with corticosteroids such as dexamethasone and betamethasone. Commensurate with the loss of LBM, fat percentage in bodybuilders who ceased training (i.e., HWT and INF) increased significantly compared to those who connected to training (CTR). This could be expected due to the immobility often accompanying a lack of training.
The lack of training led to significant reductions in both upper and lower body strengths in HWT and INF compared to CRT (Figure 3). Regarding upper body strength, the 1RM bench press was reduced by 3.30% in HWT and 6.39% in INF compared to an increase of 2.50% in CRT. In lower body strength, 1RM squat was reduced by 3.39% in HWT and 6.30% in INF compared to an increase of 2.62% in CRT. Since there is typically a direct relationship between strength and muscle mass, a major contribution to the decrease in strength can be attributed to the decrease in lean body mass. However, a decrease in neuromuscular coordination cannot be ruled out as a major factor in the strength decrease. Disser et al. (17) recently reported that one of the systems involved in COVID-19 is the muscular system, with a possibility of inflammation and muscle damage due to the virus. In the current study, some participants suffered muscle pain during and after the acute phase of the infection. Muscle pain affects muscle motor neurons and may cause reflex inhibition that could reduce muscle strength (18). In the present study, lack of exercise could be the major reason for reducing upper and lower body strength. This is supported by previous studies showing reductions of 4 - 10% in upper and lower body strengths resulting from 4 to 12 weeks of inactivity (19-21). Lovell et al. (22) believe the severity of changes during short periods of lack of training may vary depending on the initial level of fitness, individual differences in response to lack of training, and the age and sex of participants. Even a short period of lack of training in bodybuilding athletes can cause significant changes in physiological and functional capacities (23). It should also be noted that athletes' physical and mental health decline is affected by both the limitations and concerns of the quarantine period and the virus itself, especially in areas where the risks of COVID-19 and the consequent death are higher (4). Even though psychological profiles were not measured in the current participants, the mental state of those who did not train might have affected their performance. Therefore, it seems prudent to consider the mental state of athletes returning to training following a period of inactivity due to COVID-19.
The findings of this study demonstrated that after complete recovery from COVID-19, the participants had no cardiovascular symptoms. In this regard, Metzl et al. (3) reported that patients with COVID-19 who had not gone to the hospital tended to have less cardiac manifestations and could return to exercise safely. However, before returning to exercise, it is important to ensure no persistent COVID-19-related cardiac complications remains (3). In the present study, 33% of the recovered athletes had shortness of breath during exercise in the first week. Recent guidelines recommend ten days or more of rest from the onset of symptoms plus an additional seven days after symptoms resolve before returning to activity (24). Some studies demonstrated that recovered patients’ arterial oxygen saturation levels during exercise were below 88% (13). Generally, careful monitoring of respiratory symptoms and a gradual return to the activity of recreational athletes suffering from COVID-19 respiratory symptoms are essential. Pulmonary symptoms should be taken more seriously if athletes have a history of underlying lung disease.
The present study demonstrated that 50% of the recovered athletes had delayed muscle soreness in the first week. One of the most common musculoskeletal complaints of COVID-19 is myalgia and arthralgia (25). Myalgia, a common symptom in 15% of patients with COVID-19 (25), is usually self-limiting and resolves within a few days to two weeks. Like other forms of viral myositis, COVID-19 myalgia care is supportive and includes heat, ice, local analgesia, and traction. Intense exercise should be avoided in people with muscle weakness or muscle fatigue, and acetaminophen may be helpful for pain relief (3).
Current results also showed that many recovered bodybuilders experienced gastrointestinal problems during exercise. A study evaluating 116 patients with COVID-19 found that 31.9% experienced gastrointestinal symptoms, of which 22% had nausea and vomiting and 12% had diarrhea (26). Moreover, a high percentage of these patients (22%) experienced anorexia. Primary considerations for athletes with gastrointestinal manifestations as part of COVID-19 include hydration and energy availability after returning to exercise after medical treatment (26). The athletes’ fluid and calorie intake should be monitored in all symptomatic stages of the disease, and the resolving of the symptoms should be ensured after returning to sports activities.
Returning to exercise is related to the activity type and should be approximately two weeks after symptom relief (3). Participants in the current study had experienced no symptoms for two weeks and refrained from training for four weeks from the infection until their PCR test became negative. Accurate instructions for athletes’ returning to sports activities are sparse (17), but one study has recommended ten days from the symptoms’ onset (24). However, more research on athletes is needed. The exercise intensity is proposed to be about 60% in the first week to reduce the risk of injury due to lack of training and deconditioning (27).
5.1. Conclusions
This study was the first to evaluate and compare anthropometric and physiological changes due to lack of exercise in bodybuilders with COVID-19 and healthy individuals, and reported the clinical manifestations of athletes with COVID-19 on return to exercise after recovery. Considering that most athletes have resistance training workouts, it seems that the findings of this study can be useful for most athletes. However, for better results, more studies on athletes of other sports are needed. In summary, lack of training due to the closure of facilities or viral infection can reduce muscle mass and strength performance in bodybuilders. Due to possible pulmonary airway infection, it is recommended that the training of athletes who have recovered from coronavirus be closely monitored for at least two weeks to facilitate prompt medical interventions if necessary.