Introduction
Lead is a ubiquitous environmental pollutant that even in small quantities produces wide range of toxic effects in man and in experimental animals [1-3]. It is well known that lead accumulates in the skeleton throughout development and localizes in areas of bone mineralization and growth [4]. There are strong clinical evidence linking to lead exposure during intrauterine life may result in reduced birth weight, decreased preadolescent growth rates, and impaired skeletal development [4, 5]. Several in vivo and in vitro investigations showed that lead inhibits bone formation, delays growth plate chondrocyte maturation, and inhibits mineralization during ectopic bone induction [4-6]. Kennedy et al. demonstrated that lead could cause bone malformations in the rat and mouse fetuses [7].
However, the exact mechanisms underlying these effects remain unclear; many hypotheses have been given to explain lead toxicity in skeletal growth such as: impairment in the hormonal regulation of calcium absorption, displacement with calcium in the mineral bone matrix, direct effects on osteoblast function, inhibition of the vitamin D3 stimulated synthesis of osteocalcin and oxidative stress produce induction [8].
The proposed mechanism for lead induced oxidative stress is addressing by its role in the generation of ROS (Reactive Oxygen Species), plus its effect on the antioxidant defense system [9, 10]. Therefore, reducing the possibility of lead interacting with critical biomolecules and bolstering the cell’s antioxidant defenses might be associated with the beneficial role of antioxidant nutrients through exogenous supplementation of antioxidant molecules [11, 12].
Ascorbic acid (vitamin C) is a low molecular mass antioxidant that scavenges the aqueous ROS by very rapid electron transfer that inhibits lipid peroxidation [13]. In the previous works ascorbic acid supplementation in lead exposed animals significantly reduced blood, liver and renal lead levels [9-12]. Early reports found that ascorbic acid might act as a possible chelator of lead, with similar potency to that of (ethylene diamine tetraacetic acid) EDTA. Although, there has been considerable debate concerning the relationship between ascorbic acid nutritional status and heavy metal body burden in lead induced toxic effects [10, 11].
Today, interest in using of medicinal plants because of their wide range of efficacy and lesser side effects has been increased as a health aid. Garlic (llium sativum L.A) as a medicinal plant has been reported to contain two main classes of antioxidant components, namely flavonoids, and sulfur containing compounds (diallyl sulfide, trisulfide and allyl-cysteine). These are likely to play an important role in the widely demonstrated therapeutic and antioxidant effects. Besides these properties, the other efficiency of garlic is perhaps due to the presence of these sulfur-containing amino acids and compounds having free carboxyl (C=O) and amino (NH2) groups in their structures [14-16]. These biologically active compounds might chelate lead and enhance its excretion from the body resulting in reduced lead accumulation in soft tissues and blood [17].
Therefore, this work was designed in order to clarify the beneficial effects of ascorbic acid and garlic as two antioxidants during pregnancy on the bone mineralization of prenatally lead exposed rat neonates.
Results
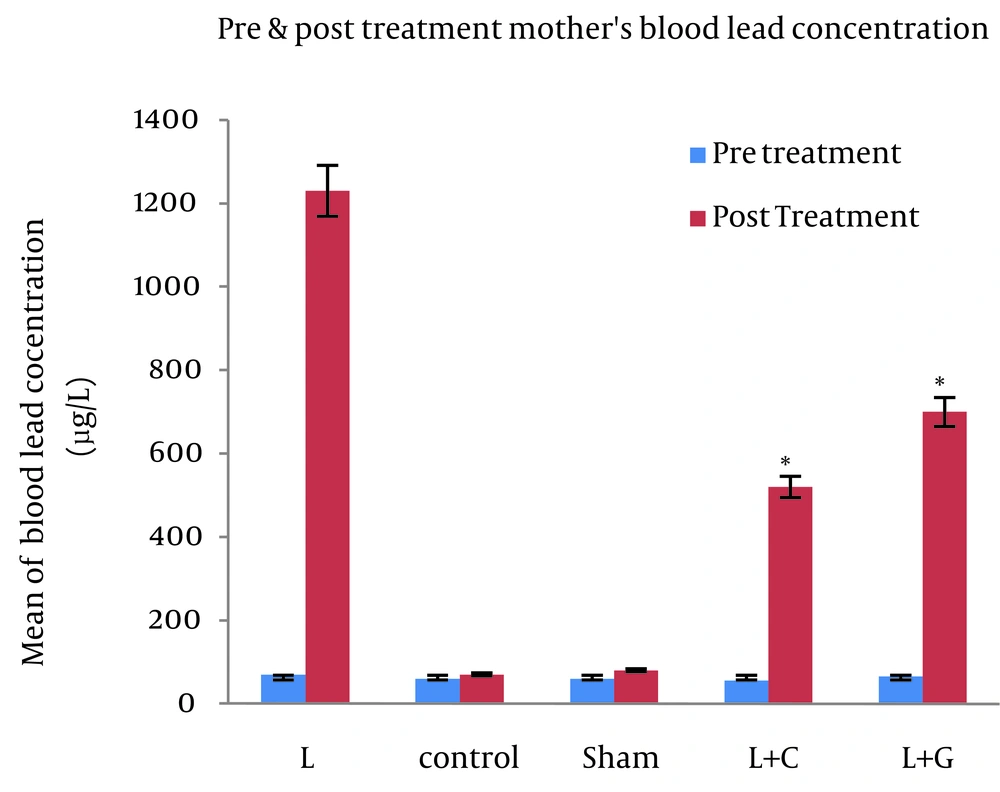
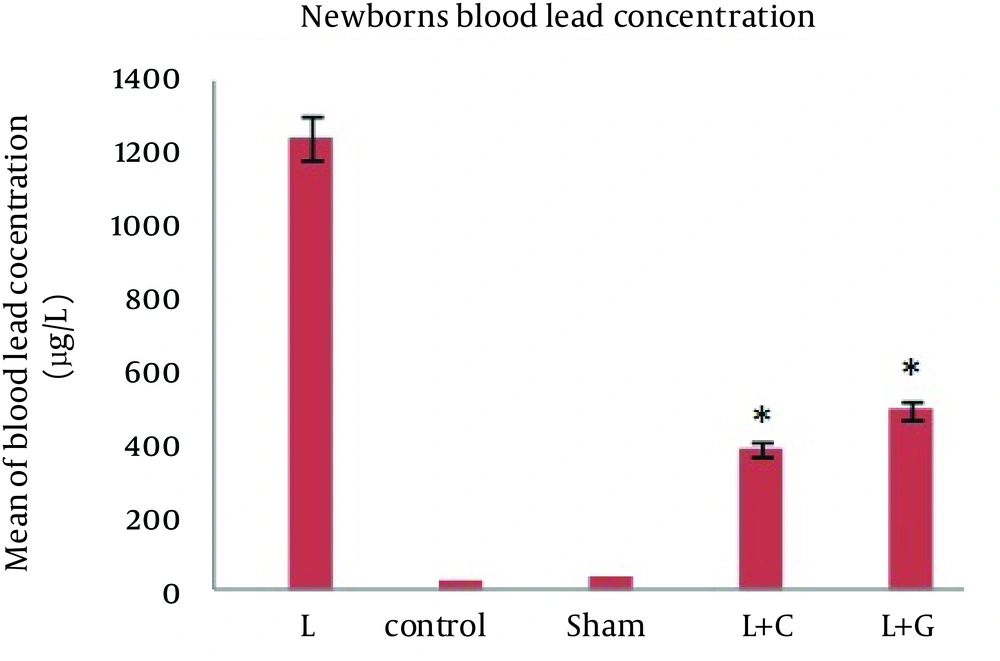
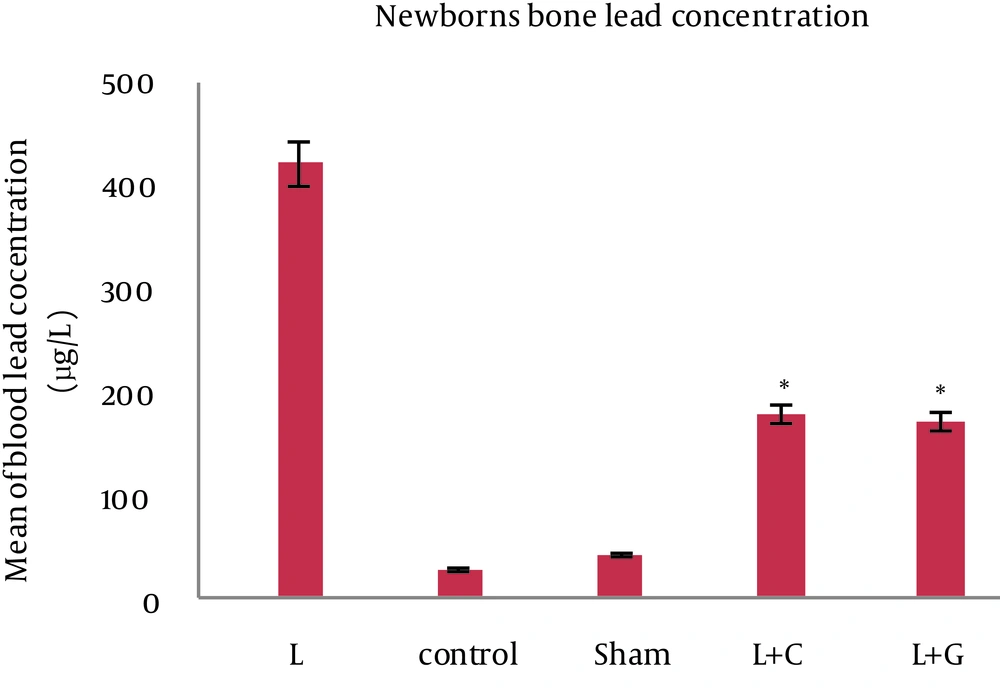
There was no significant difference in maternal blood lead levels in different groups before starting the experiments. The bone and blood lead levels were significantly higher in the lead exposed (Group L) mother and their offspring (p<0.001, Figure. 1). However, in comparison to controls, we found no significant differences in blood lead levels in mothers and their neonates that received leaded water with vitamin C (L+C) and garlic juice (L+G) (Figure. 2). The bone lead concentrations in lead exposed group born neonates also were significantly higher than that of controls (p<0.001, Figure. 3).
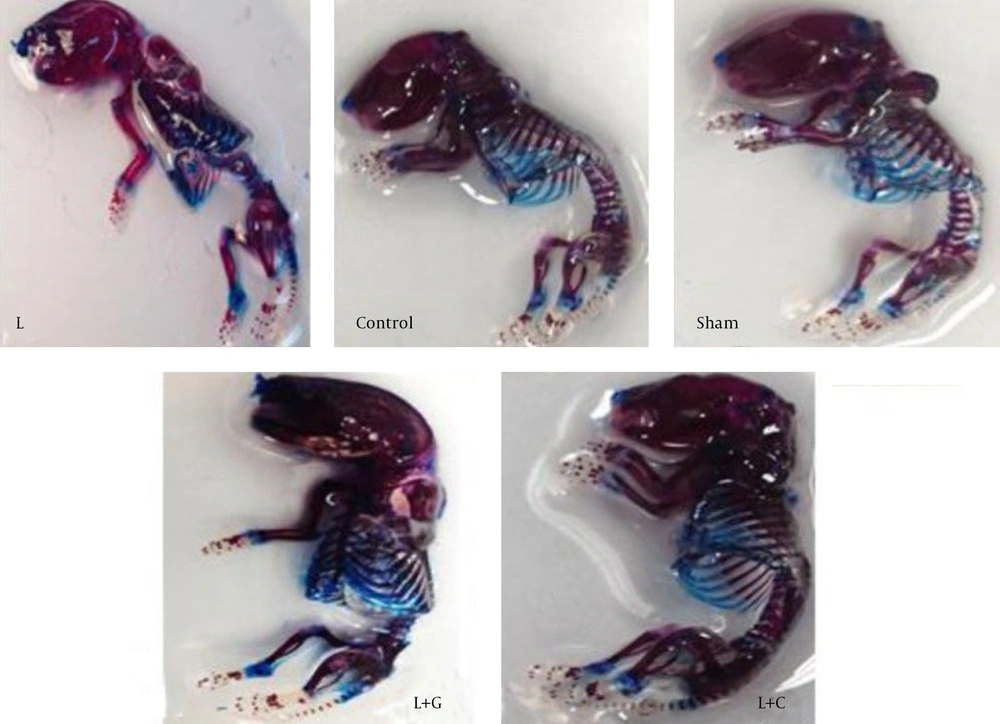
Whole embryos study: Our results demonstrated that the whole neonate staining intensity in lead exposure group was weak (pale red) comparing to other groups that was intensive (red) (Figure. 4).
Maternal blood lead concentrations of different studied groups, maternal blood lead levels are showed at the pretreatment and the post treatment; data indicate statistically significant differences between lead exposed group’s mothers with control, sham, lead+vitamin C (L+C), and lead+garlic (L+G) groups (p<0.001). Data are expressed as mean. *p<0.05 compared with lead-treated group
Blood lead concentrations of neonates in different studied groups, data indicate statistically significant differences between lead exposed group’s neonates with control, sham, lead+vitamin C (L+C), and lead+garlic (L+G) groups (p<0.001). Data are expressed as mean. *p<0.05 compared with lead-treated group
Bone lead concentrations of neonates in different studied groups, data indicate statistically significant differences between lead exposed group’s neonates with control, sham, lead+vitamin C (L+C), and lead+garlic (L+G) groups (p<0.001). Data are expressed as mean. *p<0.05 compared with lead-treated group
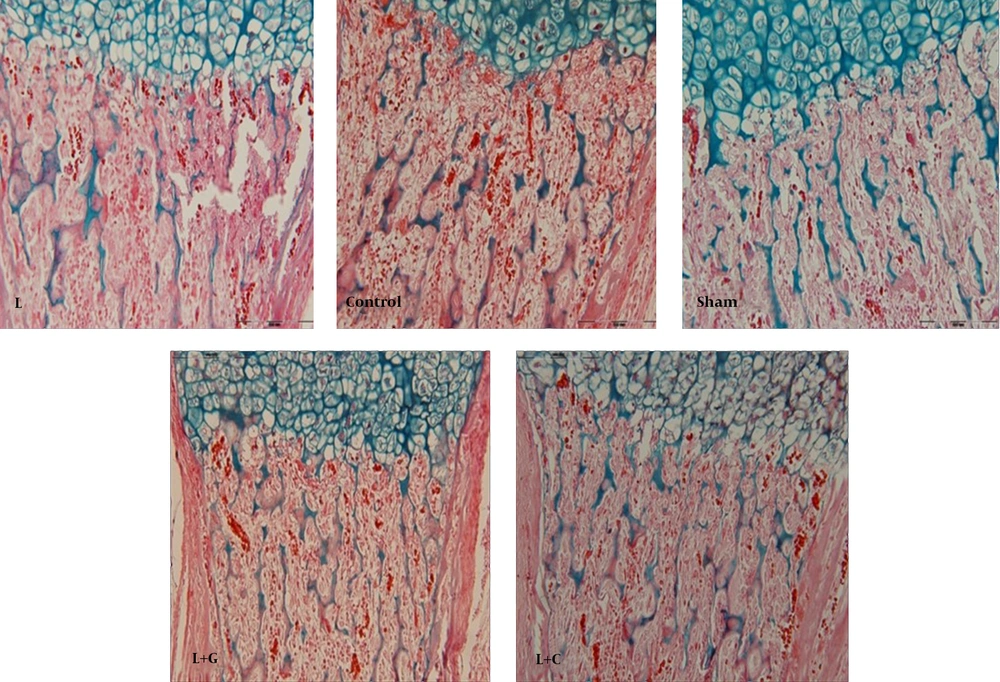
Histological Study: To assess the effects of prenatally lead exposure on bone mineralization deficits in neonates, and the beneficial effects of ascorbic acid and garlic juice consumption on this process, we used alizarin red - Alcian blue staining. Because of the bones of lead exposed group offspring showed a lower mineralization, and the cartilage tissue background was observed. Therefore, in microscopic study of bone sections, neonates’ bones of the lead exposed group demonstrated uncalcified cartilage content more than controls showing an abnormal osteogenesis and retarded bone formation in offspring born to lead exposed mothers. In contrast, the bone formation in the neonates of lead exposed group that received vitamin C (L+C) and garlic juice (L+G) showed normal ossification (Figure. 5).
Discussion
Results of this study showed that the bone and blood lead levels were significantly higher in the lead exposed mother and their offspring. There was no significant difference in blood and bone lead levels in mothers and their neonates that received leaded water with vitamin C and garlic juice. In microscopic study of neonates bones in lead exposed group demonstrated uncalcified cartilage content more than control group. In contrast, the bone formation in the neonates of lead exposed group that received vitamin C and garlic juice showed normal ossification.
Previous human and animal investigations indicated that the prenatally lead exposure suppresses growth processes. An important element of somatic growth is skeletal growth.
The fetal skeleton is a useful indicator of embryonic development and frequently reflects changes in the maternal-fetal environment [21, 22]. Aliverti et al. studied the number of ossification centres in different skeletal districts of rat fetuses, and suggested that the stage of skeletal ossification can be used in teratogenic studies in the rat to evaluate retarded fetal development [22]. The degree of growth retardation also was found to differ among ossification centers [23].
The results of present study indicated the leaded water consumption could successfully elevate the whole blood lead concentrations in both mothers and their neonates. Because lead crosses through the placental barrier freely, it will be logical if maternal lead exposure during pregnancy increases lead levels in neonates. Our results confirm the previously human and animal studies reported findings that maternal lead burden is an important determinant of infant lead levels. It is necessary to note that this observation was relevant to human pregnancy because most women are likely to have limited exposure to lead during pregnancy, but may have considerable body lead burdens from a history of lead exposure, including exposure during childhood [23-26].
Our data also showed significantly higher lead contents in bone of neonates born to lead exposed rats than that of controls. It is well documented that lead has the affinity to accumulate in the bone throughout development, and has direct and indirect effects on growth. The mechanisms behind lead effects on growth seem to involve actions at different sites. Lead may affect directly on osteoblast function, interfere with vitamin D metabolism or with calcium’s role as a cellular messenger in its endocrine functions, or it may act as a depressant of food intake [6, 8, 23, 24].
The histological results in our study showed an apparent defect in ossification of growth cartilage plates in neonates that exposed with lead in prenatal period. These observations add to the recent accumulating evidence suggesting that intrauterine lead exposure inhibits bone formations in experimental animals. Many previous studies have shown that the lead localizes in areas of bone mineralization and can cause bone malformations in the rat and mouse fetuses, delay growth plate chondrocyte maturation, inhibit bone formation in dogs, and inhibit mineralization in vivo during ectopic bone induction [6, 7, 24].
The present study results indicated that the lead concentrations of whole blood and bone in neonates born to lead exposed rats that received ascorbic acid and garlic juice showed insignificantly difference when compared to controls. Moreover, we can not found any histological changes in bone formation in neonates born to lead exposed rats that received ascorbic acid and garlic juice. In agreement with the other studies, these results demonstrated that antioxidants (i.e. ascorbic acid and garlic) may prevent the lead cross from placental barrier, and this role may be reflect in their preventive effects on bone malformation.
Recent studies have suggested oxidative stress as one of the important mechanisms of toxic effects of lead. So, antioxidant defenses have been found to be defective in many of the disturbances caused by ROS production [10, 25]. Ascorbic acid is considered one of the most prevalent antioxidative components of fruits and vegetables that interact directly with the oxidizing radicals. Ascorbic acid also is essential in collagen formation and ascorbic acid deficiency results in an abnormal extracellular matrix of the connective tissues, and a disrupted endochondral ossification [26].
Garlic is also a good source of phytochemicals with proven antioxidant properties. The biological activity of garlic may be mediated by its organic allyl sulfur components (mainly Allicin). It has been suggested that the biological activities of these ingredients may be related to their thiol modification and antioxidant characteristics [26].
In conclusion, the present study demonstrated that consumption of both ascorbic acid and garlic juice throughout pregnancy has beneficial roles on the lead induced toxicity on rat neonate's bones. These supplements had preventive effects not only in elevated blood and bone lead concentrations but also in bone formation deficiencies. Although, this idea need to more investigations, the authors recommend the consumption of plenty of raw garlic within the food as well as utilization of fresh fruits rich of ascorbic acid, especially for pregnant women living in lead contaminated areas such as industrial and central urban points to protect themselves and their offspring against any expected harms such as skeletal defects.