1. Background
Anabolic steroid hormones are often used to cure various diseases (1). Testosterone, known as the ancestor of most steroids, was first discovered by Adolf Butenandt et al., and since then, this hormone has been applied without any legal limitation to treat specific diseases and to strengthen general and particular body muscles (2). Moreover, athletes sometimes abuse this hormone to improve their performance (3). Statistics show that 67% of athletes in the USA had used anabolic-androgenic steroids in 2007 (4). Improper consumption or abuse of these medicines causes long-term and irrecoverable damage to some vital body organs like the heart, liver, brain, and kidneys (5). Surprisingly, a survey in 2006 showed that only 38% of interviewed athletes were aware of the side effects of anabolic steroids (6). The medicinal type of anabolic steroids, known as testosterone enanthate, is a natural androgen hormone, and in recent years, its consumption has been widely increased among athlete women working in strength sports (7). This hormone affects the cell nucleus through complicated interactions, spreads in a cell due to its solvability in lipids, enters the cell nucleus in combination with proteins, and regulates the production of several proteins (8).
In the past, anabolic-androgenic steroids had been prescribed to cure anemia in patients suffering from chronic kidney disease (CKD). However, their dosage to cure anemia was much less than their dosage in doping (9). Prescribing androgens to cure anemia in patients with CKD was stopped considering its numerous destructive effects on kidneys (10). Studies show that steroid abuse is closely related to kidney failure (11). Daher et al. demonstrated that the long-term consumption of high-dose anabolic-androgenic steroids led to renal damage, nephrotic inflammation, and tubular necrosis (12). In addition, Almukhtar et al. reported that the kidney tissues of four bodybuilders who used more than 400 mg of testosterone propionate in a week showed tubular necrosis and increased kidney inflammation (13). Apoptosis, an influential factor in developing kidney fibrosis, can be triggered by various inflammatory cytokines (14). Androgens also play an essential role in the induction of apoptosis. For example, androgens can induce kidney cell apoptosis by inducing a caspase-dependent apoptotic pathway (15). Also, testosterone can induce the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), leading to renal inflammation and CKD progression (16).
A study by Castaneda et al. clarified that moderate resistance training reduced the levels of inflammatory factors in the kidney tissue (17). Professional bodybuilders and powerlifters utilize androgenic-anabolic steroids to increase their performance and muscle mass and decrease body fat (18). Using androgenic-anabolic steroids has increasingly propagated among bodybuilders and non-competitive athletes who seek increasing muscle mass (19). Androgenic-anabolic steroids in females may lead to menstrual irregularity, clitoris hypertrophy, and uterus and breast atrophy (20). Moreover, despite the popularity of testosterone enanthate consumption among women, no study has yet evaluated changes in the inflammatory factors in the kidney tissue following testosterone enanthate consumption.
2. Objectives
Here we assessed if eight weeks of resistance exercise in combination with testosterone enanthate injection could influence inflammatory factors in the renal tissue of female Wistar rats.
3. Methods
3.1. Animals and Research Design
This experimental study was conducted on 21 female Wistar rats aged eight weeks on average and weighed 200 ± 20 g. The rats were randomly divided into three groups: control (n = 7), training + placebo (olive oil) (n = 6), and training + testosterone enanthate (n = 8). The rats were placed in polycarbonate cages (four animals per cage) and kept at a 12-hour dark/12-hour light cycle, 22 ± 2°C, and 45% ± 5% humidity. The rats were provided with water and food ad libithium.
3.2. Ethical Considerations
In this study, all ethical principles published by the National Institute of Health (NIH) on working procedures with animals (e.g., food and water accessibility, suitable care conditions, imposing no obligation to do the resistance training, and being sure of animal anesthesia before autopsy) were closely watched. The exercise would be stopped during resistance training if the animal slipped while climbing the ladder with weights.
3.3. Research Protocol
The 8-week resistance training protocol included climbing a 1-meter ladder with 26 steps at a steep of 85° with weights being carried on the rats’ tails. The protocol consisted of five days of training and two days of rest in a week (each session consisted of four sets of six repetitions with 60 to 90 seconds of rest). The weights were increased during the study. In the first week, the load weight was equal to 40% of the rats’ weight and was increased by 20% per week till the fifth week. In the fifth week, the weight was reduced by 20%, but in the sixth week, it was increased by 40% of the rats’ weight. In the final two weeks, the load weight was increased by 20% of the rats’ weight per week (21). The rats were weighed two times a week (Table 1). The rats in the testosterone enanthate group were injected with 20 mg of testosterone enanthate three times a week (21).
| Groups | Time | |||||||
|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | |
| Intensity (%) | 40 | 60 | 80 | 100 | 80 | 120 | 140 | 160 |
| Repetition | 6 | 4 | 5 | 6 | 4 | 4 | 5 | 6 |
| Sets | 1 | 2 | 2 | 2 | 2 | 4 | 4 | 4 |
| Rest between repetitions (s) | 15 | 15 | 30 | 30 | 15 | 30 | 30 | 30 |
| Rest between sets (s) | 0 | 60 | 90 | 90 | 60 | 100 | 120 | 120 |
Resistance Training Protocol
3.4. Laboratory and Statistical Methods
At the end of the 8-week procedure, the animals were fasted of food and water for 12 to 14 hours. Tissue samples were collected 48 hours following the last training session and testosterone injection. Anesthesia was performed using a combination of ketamine (30 - 50 mg/kg) and xylazine (3 - 5 mg/kg) through intraperitoneal injection. After 40 to 50 seconds of administration, the animals were in a suitable anesthesia condition. According to a predefined timing, renal tissue sampling was performed in all three groups (i.e., control, training + placebo, and training + testosterone enanthate). After being washed with physiologic serum, the samples were immediately placed into special tubes. The tissues obtained were immediately frozen in liquid nitrogen (-196°C) and then kept at -80°C in the laboratory until further evaluation.
3.5. Gene Expression Analysis
The gene expression of IL-6 and TNF-α in the kidney tissue was determined using real-time PCR. For this purpose, an RNA extraction kit (Cat. No., DQ383-40h), a cDNA synthesis kit (Cat. No. BR441-096, BioFACT TM, 5X RT Pre-Mix), and 2X Real-Time PCR Master MixTM (BioFACT® h) at a final concentration of 2.5 mM for MgCl2, including SYBR Green I and w/o Rox reference dye, were used.
3.6. Statistical Analysis
To analyze the data, in addition to descriptive statistics (mean ± SD) to present the variables, the Shapiro-Wilk test was used to assess the normality of data distribution, after which the Leven test at a P < 0.05 significance level was applied to examine variance homogeneity. Then one-way ANOVA and the Bonferroni post-hoc test were utilized for intergroup and pair-wise comparisons, respectively. All statistical procedures were performed in SPSS software, version 20. Graphs were drawn using the GraphPad Prism (9.4.0.673) software.
4. Results
Table 2 represents the mean and standard deviation of TNF-α and IL-6 levels in female rats in the three study groups (i.e., control, training + placebo, and training + testosterone enanthate), along with the results of comparing groups using the ANOVA and Bonferroni tests.
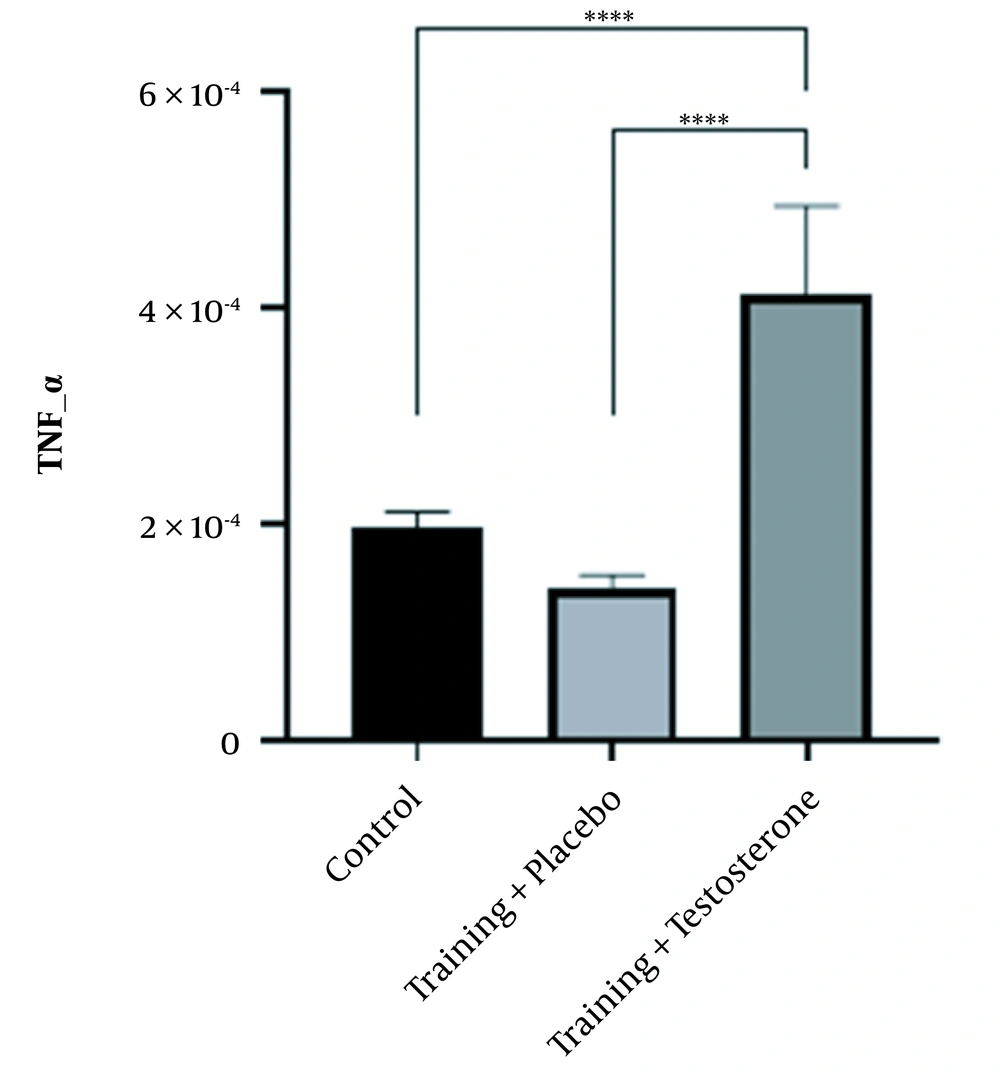
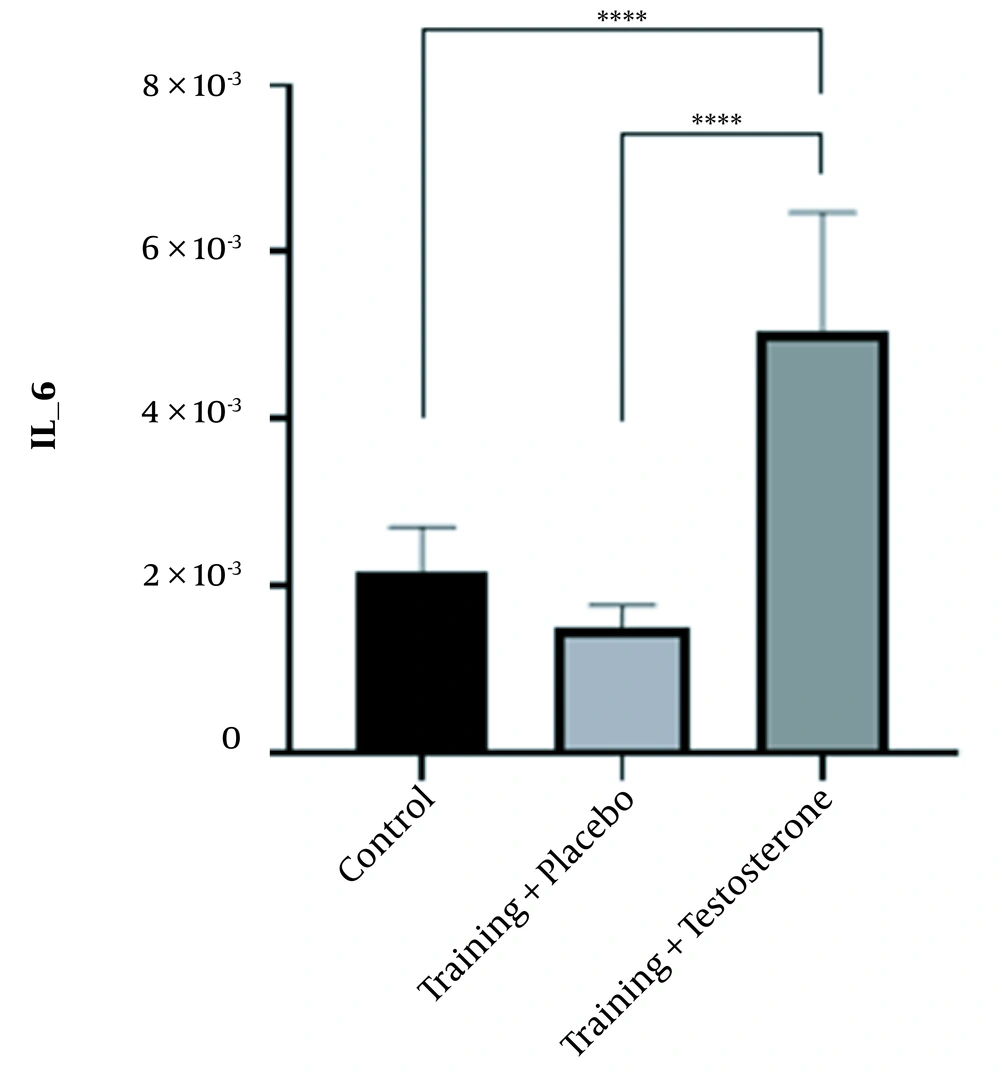
4.1. Effect of Testosterone Enanthate Plus Resistance Training on TNF-α and IL-6 Genes’ Expression Levels in the Kidney Tissue of Female Wistar Rats
The ANOVA test clarified that treatment with testosterone enanthate led to significant changes in TNF-α (F (2, 18) = 57.093, P < 0.0001) and IL-6 (F (2, 18) = 29.240, P < 0.0001) levels in the kidney tissue of female rats. Paired comparison using the Bonferroni post-hoc test revealed that the mean differences in TNF-α (mean difference(j-i) = -0.56E-4, P = 0.205) and IL-6 (mean difference(j-i) = -0.65E-3, P = 0.693) levels were small and insignificant between the control and training + placebo groups. However, the mean difference in renal TNF-α level was considerable and statistically significant between the training + testosterone and control groups (mean difference(j-i) = 2.18E-4, P < 0.001), as well as between the training + testosterone and training + placebo groups (mean difference(j-i) = 2.74E-4, P < 0.001) (Figure 1). Likewise, the mean differences in renal IL-6 levels between the training + testosterone and control groups (mean difference(j-i) = 2.91E-3, P < 0.001), as well as between the training + testosterone and training + placebo groups (mean difference(j-i) = 3.56E-3, P < 0.001) were remarkable and statistically significant (Figure 2).
In summary, it can be concluded that the supplementation of testosterone led to a significant increase in the levels of inflammatory factors in rats compared to control and placebo groups. Additionally, performing resistance exercises by the placebo group did not have a significant impact on the levels of inflammatory factors compared to the control group.
5. Discussion
After eight weeks of resistance training and testosterone enanthate injection, IL-6 and TNF-α were measured in rats’ kidney tissues. The results demonstrated that resistance training alone led to an insignificant reduction in the inflammatory cytokines assessed while consuming testosterone, either alone or in combination with resistance training, significantly increased the levels of IL-6 and TNF-α inflammatory factors. Castaneda et al. showed that 12 weeks of moderate resistance training reduced IL-6 levels in patients suffering from CKD (17). As an influential factor, resistance training can direct inflammatory factors toward the kidney tissue (15). We also observed that although resistance training could reduce renal IL-6 and TNF- α, the decline was not significant, which can be attributed to the differences among the subjects. The level of primary inflammation differs between healthy and functionally impaired kidneys due to chronic diseases, justifying the reduction observed in the levels of inflammatory factors (22).
In the present study, a significant increase in IL-6 and TNF-α was observed in the rats simultaneously receiving testosterone supplementation and resistance training. Our results in this study were consistent with that of Liborio et al. and Daher et al. (12, 22). As an explanation, it can be said that testosterone may have a role in inducing the production of inflammatory cytokines such as IL-6, TNF-α, and IL-1 (16, 17). The overexpression of TNF-α leads to its binding to the receptor type 1, causing an inflammatory response and cell proliferation. In this process, NF-kb is activated and induces the transcription of the genes involved in cellular survival and proliferation. In order for NF-kb to be activated, after the binding of TNF-α to the receptor type 1, TNF-receptor-associated factor 2 (TRFA-2), which is associated with the TNF-α receptor, binds to TRADD, followed by the recruitment of receptor-interacting protein (RIP) to the complex. Finally, both RIP and TRAF-2 contribute to the activation of the inhibitor of nuclear factor-kB (IKB) kinases. These kinases phosphorylate IkB and release NF-Kb, and NF-Kb triggers the transcription of the genes involved in inflammation, such as endothelial adhesion molecules, IL-6, and other inflammatory mediators. So, TNF-α invigorates its expression by activating NF-Kb, and these cytokines lead to renal inflammation and the progression of CKDs (23). In addition, inflammatory cytokines like TNF-α and IL-6 can augment the activity of androgen receptors (12). Metcalfe et al. demonstrated that in male rats, TNF-α increased proptosis, profibrotic signaling, tubular interstitial fibrosis, and kidney dysfunction, accompanied by endogenous testosterone production in female rats, which could be alleviated by the administration of exogenous testosterone (11).
Our results were inconsistent those of Mohamad et al. (24), which may be due to differences in testosterone dosage and subjects’ characteristics. Patil et al. conducted a study on male rats and reported that a lower dose of testosterone protected kidneys against ischemia-reperfusion injury; however, a higher dosage induced the overexpression of TNF-α in the kidney, negating a renal protective effect (25). Therefore, TNF-α is a critical cytokine involved in ASS-related renal injury (25). Loh et al. declared that the increased expression of aquaporins 1 and 7 in the complex proximal tubule and aquaporins 2, 4, and 6 in collecting ducts caused a testosterone-dependent elevation of the arterial pressure in rats (26). These findings indicated that ASS could elevate water reabsorption and, subsequently, blood pressure in patients with CKD (24).
Another critical issue that should be considered in relation to the inflammatory responses associated with testosterone is the difference between males and females in their reactions to hormonal mediators (24). Zhao and Schooling reported that testosterone consumption caused more kidney injuries and related diseases in women than in men, while consuming a suitable dose of testosterone may even avoid CKDs in men (27). The exact mechanism of this phenomenon is unknown, but it may be attributed to the dominant role of testosterone in men’s reproduction and its compatibility with male physiology.
5.1. Conclusions
According to our results and former studies, enanthate steroids can be an essential factor in inducing renal inflammation, evidenced by kidney damage observed in animal models and even bodybuilders consuming steroids. Animal cells can be suitable replacements for expressing metabolic characteristics in humans. Therefore, experiments in female rats can provide valuable information with regard to human beings. As a result, women athletes must be aware of the harmful and sometimes unrecoverable effects of testosterone enanthate, which is consumed to increase performance, gain weight, and build muscles.