1. Introduction
Pulmonary atresia is one of the congenital heart abnormalities that is usually diagnosed shortly after birth. In this disorder, the tricuspid valve between the right atrium and the right ventricle, which carries blood from the heart to the lungs (pulmonary valve) in the neonate's heart, is not fully formed. Without therapeutic intervention, this lesion will have a high mortality rate (1). About 50% of neonates with this congenital disease show symptoms on the first day of birth and 80% within the first month. Risk factors of this disease include Down syndrome, poor blood sugar control in diabetic patients, heavy alcohol consumption during pregnancy, and a family history of heart disease (2).
Under normal conditions, blood enters the right ventricle and lungs through the tricuspid valve. Ductus arteriosus is an embryonic vascular connection between the pulmonary artery and the aorta, which blood from the pulmonary pathway to the systemic circulation during the fetal period directly and usually closes within 72 hours after birth. Anatomic closure of this duct usually occurs in the first week after birth (3). In infants with pulmonary atresia, blood through patent ductus arteriosus (PDA) circulates in the lung. If this duct is closed, the oxygenation of the neonate will be disturbed and cause death (4). In most premature neonates, with the pulmonary artery pressure drop after birth and non-contraction of the ductus arteriosus, the shunt of a large volume of blood from the ductus arteriosus causes an increase in pulmonary blood flow and a decrease in systemic blood flow. Neonates are exposed to complications such as intracerebral hemorrhage, pulmonary hemorrhage, and necrotizing enterocolitis due to reduced systemic circulation (5).
The primary treatment for pulmonary atresia is the use of prostaglandin E1 for keeping the ductus arteriosus open and establishing pulmonary blood circulation in neonates, which, if not used, the ductus arteriosus closes within 2 to 3 days after birth and leads to death (6). The final and complementary treatment for these neonates is mainly surgical treatment. According to the patient's age, surgical treatment methods that are currently used for these people include balloon septostomy, modified Blalock-Taussig (BT) shunt between the aorta and pulmonary artery, pulmonary artery bands, Fontan procedure, and Glenn shunt method to connect the superior vena cava to the artery (2, 7). Patent ductus arteriosus stenting is a solution for the primary treatment of neonates with reduced pulmonary blood flow, such as pulmonary artery atresia, tricuspid valve atresia, and single ventricle neonates, and also a treatment solution to increase systemic blood flow (in disorders such as severe coarctation of the aorta and hypoplastic left heart syndrome (HPLS) (8). Rashkind's atrial septostomy (creating an inter-atrial hole as a solution for bilateral mixing of pulmonary and systemic blood and arterial oxygen saturation) is a palliative interventional method in certain forms of congenital heart diseases, including patients with intact ventricular septum, pulmonary artery atresia, and hypoplastic right ventricle (single ventricular) (9, 10). This study aims to report a case of embolized stent and Rashkind septostomy that suffered hemolysis due to stent in a 2-day neonate with pulmonary atresia and single ventricle. This case report has been approved by the ethics committee of Rafsanjan University of Medical Sciences with the ethics code IR.RUMS.REC.1401.050.
2. Case Presentation
The patient was a 2-day-old neonate boy with a gestational age of 35 ± 2 weeks and birth weight of 2800 grams, who was delivered by cesarean section from a 22-year-old mother. Neonate's vital signs after birth were abnormal, and during the examination, cyanosis, tachypnea, grunting, and respiratory distress were observed. Auscultation had a systolic murmur at the left upper edge of the sternum. O2 saturation of the newborn was about 70%, with a nasal oxygen intake of 3 L/min. The results of the neonate's X-ray showed pulmonary vascular anemia, and echocardiography reported the presence of a single ventricle disorder (hypoplastic right ventricle) with an intact interventricular septum, pulmonary artery atresia, intact ventricular septum, severe tricuspid valve insufficiency, and PDA. To keep open the PDA, the neonate was treated with prostaglandin E1 (in the form of PGE1 = 0.1 µg/kg/min), and a day later, after endotracheal intubation, the neonate was angiographed for PDA stenting and Rashkind's inter-atrial septostomy operation.
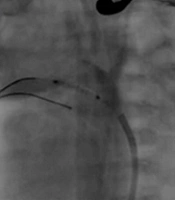
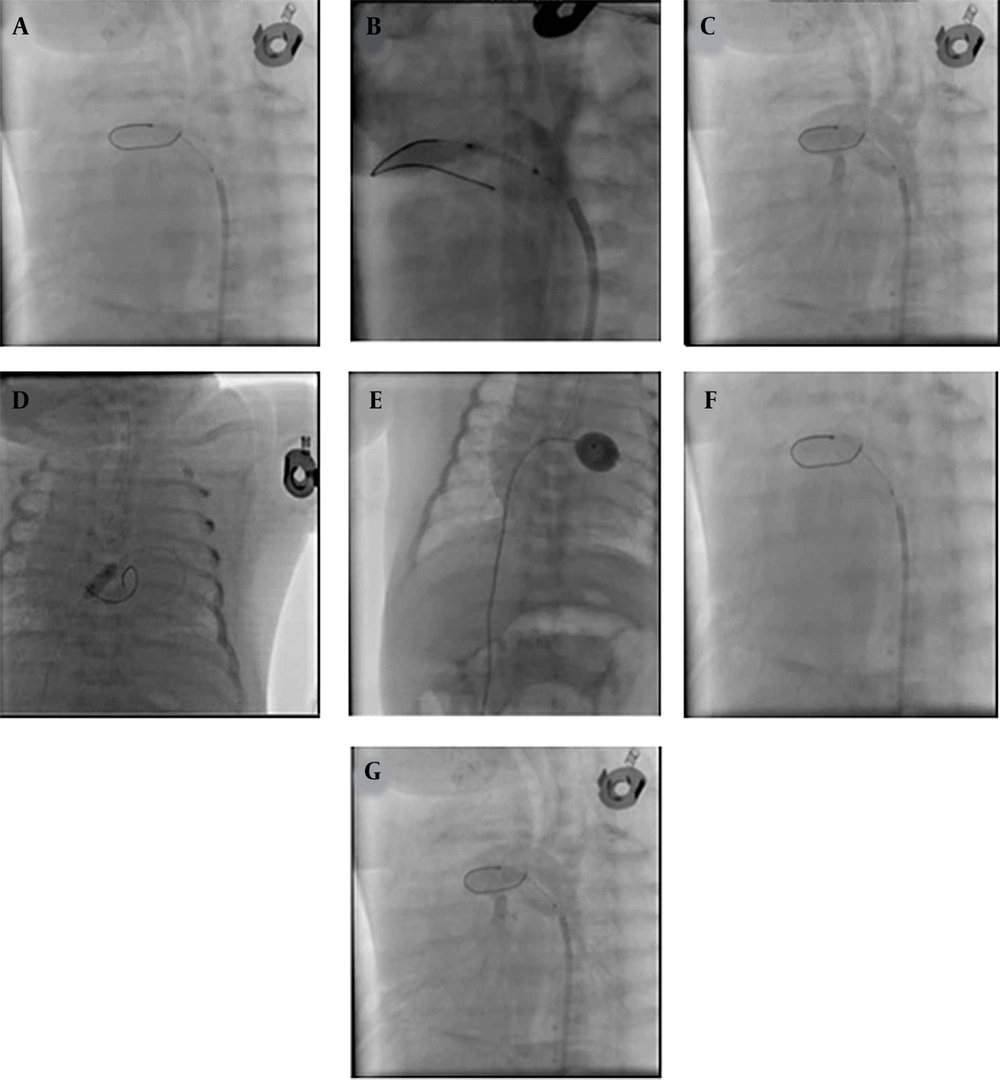
Patent ductus arteriosus stenting (with XincAlpine 12*3 coronary stent) was performed initially. However, during Rashkind's inter-atrial septostomy operation with Z five balloon, the neonate suffered a cardiac arrest, and the neonate was immediately resuscitated by performing cardiopulmonary resuscitation (CPR). Angiography examination indicated embolization of the PDA stent to the branch of the pulmonary artery, and the attempt to retrieve the stent was unsuccessful. Finally, it was decided to place a larger stent (4 × 16 coronary stent) in the PDA, which was successfully performed (Figure 1). The blood oxygen saturation reached 85%, and the neonate was transferred to the neonatal intensive care unit (NICU). Then, from the second day, the neonate had severe hematuria, and the patient's blood test results indicated high lactate dehydrogenase (LDH), reticulocytosis, and anemia due to hemolysis. The neonate's hemolysis was reduced within 2 weeks with conservative treatment (cell pack, rehydration, electrolyte modification, and urine alkalinization), which reduced LDH, stopped hemolysis, cleared the urine, and the neonate was discharged in good general condition. The infant is now 5 months old and is on the single ventricle surgery list (Fontan procedure and Glenn shunt method).
Stenting patent ductus arteriosus (PDA) and embolized smaller stent, Rashkind procedure, and second stenting PDA with a larger stent. The following septostomy guides: A, aortogram at Lateral showed: PDA tubular and pulmonary atresia; B, PDA stenting was done with coronary stent Xine Alpine size 3*12; C, aortogram at lateral showed stent PDA had a good place; D, septostomy Rashkind was done first with a small coronary balloon; E, septostomy was done with Z five balloon size 9; F, aortogram at lateral showed embolized stent in distal branch pulmonary artery; G, second stenting PDA was done with coronary stent 4 × 16 successfully.
3. Discussion
Balloon opening of the interatrial septum in congenital heart diseases was introduced by William Rashkind several decades ago (10). Septostomy is essential to improve arterial and venous blood mixing, especially in neonates with vascular displacement. This method is one of the emergency cases in neonatal cardiology performed as a catheterization laboratory (11). The present study performed PDA stenting and Rashkind balloon atrial septostomy after the initial treatment measures. Given that the patient then suffered from cardiopulmonary arrest and the angiographic results indicated PDA stent embolization in the pulmonary artery branch, a larger stent was successfully implanted in PDA, and arterial oxygen saturation increased. These treatment methods eliminate the need for open surgery and shunt placement in neonates with PDA and single ventricles, and this intervention can be performed in the first hours of birth.
Balloon atrial septostomy is a primary life-saving treatment, especially for congenital cyanotic heart defects. This procedure aims to open the interatrial septum, which increases the bilateral mixing of pulmonary and systemic venous blood and thus improves oxygen saturation in this type of heart defect. Although the use of this method has complications such as arrhythmia, heart block, myocardial ischemia, and coronary artery stenosis or obstruction (9), which indicates the need to pay attention to the patient's symptoms after stenting, in cases where the signs and results of angiography indicate the presence of stent embolization, a larger stent can be inserted with necessary caution to treat the disorder. This measure can save the patient's life and improve his/her general condition until single ventricular surgery is performed.
3.1. Conclusions
Identifying and carrying out therapeutic interventions in neonates with PDA, intact ventricular septum, and severe tricuspid regurgitation at the beginning of birth is very important for the patient's health. Diagnosing this disease in the 18th week of pregnancy is possible using a fetal ultrasound. Keeping the PDA open with a proper stent and creating a Rashkind atrial septostomy increases the two-way mixing of pulmonary and systemic venous blood, improves the baby's oxygen saturation, and eliminates the need for surgery.