1. Background
Arctium lappa L., commonly known as burdock, is a member of the Compositae (Asteraceae) family, that has been widely used in folk medicine in different regions of the world including North America, Australia, Middle East, Russia, China, Japan, India [1]. The various parts of burdock such as roots, seeds and leaves are documented to possess various medicinal properties.
Pharmacological studies and clinical trials indicated that burdock roots have hepatic-protective properties [2], anti-inflammatory and antioxidant activity [3], ability to remove heavy metals from body [4], antidiabetic activity [1], and suppressive effect on cancer cells [1, 5]. Burdock leaves also has been used therapeutically for treatment of bacterial infections [6], attenuating excessive nitric oxide (NO) generation at inflammatory sites [7], and improvement of skin problems in eczema, acne and psoriasis [1].
Despite extensive experimental investigation into the therapeutic effects of burdock; however, few studies have evaluated its effect on immune responses. Suppression of allergic response by inhibition of degranulation and release of cysteinyl leukotrienes from peripheral blood mononuclear cells [8], inhibition of IgE secretion from human B cell line [9], and decreased NO production from macrophages [7] are major findings related to immunomedulatory properties of burdock.
Various immunomodulatory compounds such as tannin, arctiin, arctigenin, lappaol F, caffeic acid, and etc. isolated and purified from different tissues of the burdock plant [9-12]. Ferracane et al. showed that amount of these compounds varies greatly between different tissues of burdock plant [13]. Accordingly, do root and leaves of burdock have similar or different immunomodulatory properties?
To answer this question, in this study, at first, we prepared hydroalcoholic extract of burdock root and leaves. After that, we evaluated and compared the effects of different concentration of root extract of burdock (REB) and leaves extract of burdock (LEB) on mitogen-stimulated splenocytes proliferation, Th1/Th2 cytokine secretions, and NO production by LPS- stimulated macrophages in vitro.
2. Methods
2.1. Extract Preparation
In this experimental study, Burdock was collected from the mountains in North Khorasan province (Iran) in April and May 2016. Plant was identified and authenticated by Dr. Sheykh, Medicinal Plants Research Center, School of Medicine, North Khorasan University of Medical Sciences. Root and leaves of plant were dried in shade and powdered with an herbal grinder. For preparation of hydroalcoholic extract, 50 g of powdered root or leaves were macerated with 500 mL 50:50 EtOH/H2O for 48 hours. After that the extract was shaken, filtered, and supernatant evaporated in a rotating evaporator (Buchi, Flawil, Switzerland) under reduced pressure until dryness. The yields of REB and LEB were ranged from 8% to 10%. The amount of different components of REB and LEB were analyzed using HPLC. Profile of the compounds of REB and LEB were similar with previous studies (data not shown) [13, 14].
2.2. Animals
Thirty-two 6 - 8 week-old female BALB/c mice were purchased from Razi institute (Mashhad, Iran). They were given ad libitum access to standard mouse chow and water. The experimental protocols used in this study were approved by Animal Ethics Committee of North Khorasan University of Medical Sciences, Bojnord, Iran.
2.3. Isolation and Culture of Macrophages and Splenocytes
Isolation of mouse peritoneal macrophages from female BALB/c mice was carried out according to the procedure described by Bibak et al. [15]. Briefly, murine peritoneal cells were harvested by lavage of the peritoneal cavity with 10 mL of RPMI 1640 (Invitrogen, Germany). The cells were centrifuged at 200 g, washed and cultured in petri dishes at 37°C for 4 hours. After removing non-adherent cells by washing, the trypsinized cells were adjusted to 1 × 106 cells/mL in complete RPMI medium (RPMI medium with 10% FCS (Invitrogen, Germany), and 50 IU/mL penicillin/streptomycin) (Sigma-Aldrich, USA). The isolated cell suspensions of macrophages also were used for our next experiments.
Spleens from BALB/c female mice were removed and washed with sterile Hanks’ balanced salt solution (HBSS) (Sigma-Aldrich, USA).Spleens were finely minced with scissors, and passed through a 70 µm mesh. After washing twice in PBS, erythrocytes were removed using 0.83% NH4Cl (Merck, Germany), and after washing the remaining cells were suspended in RPMI 1640 medium with 10% FCS.
2.4. Effects of REB and LEB on Mitogen-Stimulatedsplenocyte Proliferation
To investigate the immunomodulatory effects of REB and LEB, splenocytes were plated on a 96-well flat-bottom plates at a density of 1 × 106/mL (200 μL per well) and cultured in complete RPMI 1640 medium. Cells were stimulated with phytohaemagglutinin (PHA) (Baharafshan, Iran) 10 μg/mL or LPS (Sigma-Aldrich, USA)5 μg/mL in the presence or absence of different concentrations of the ethanolic extracts (1, 10, 20, and 50 µg/mL) at 37°C with 5% CO2 for 72 hours. The splenocytes cultured in complete RPMI medium and treated with PBS, served as the negative control group.
After the incubation time, cell-free supernatants were collected and stored at -70°C for measurement of IL-4 and IFN-γ by ELISA. Subsequently, cell proliferation was measured based on the MTT assay [16]. The absorbance of each well was determined at 540 nm. Proliferation activity was reported as stimulation index (SI) which was calculated according to the following formula: OD 540 nm of stimulated wells/OD 540 nm of un-stimulated wells.
2.5. Cytokine Assay
The concentrations of IL-4 and IFN-γ cytokines in the supernatant of cultured splenocytes were measured by sandwich ELISA according to the instructions of the manufacturer (eBioscience, USA). The sensitivity of the ELISA kits for IL-4 and IFN-γ were 4 pg/mL and 15 pg/mL, respectively.
2.6. Treatment of Peritoneal Macrophages with REB or LEB
To evaluate the effects of REB or LEB on the activity and nitric oxide (NO) production by macrophages, on each well, 2 × 105 cell suspensions (200 µL/well on 96-well flat-bottom plates) were treated with or without LPS (10 µg/mL from Escherichia coli O111: B4, Sigma, USA). Next, different concentrations of the ethanolic extracts (1, 10, 20, and 50 µg/mL) were added to wells and cells were cultured at 37°C and 5% CO2 for 48 hours. Macrophages cultured in complete RPMI medium and treated with PBS, served as the negative control group. LPS-treated macrophages cultured in complete RPMI medium, were used as positive control group. In addition, complete RPMI medium was used as the blank control group.
After 48-hours incubation cell, culture supernatants were collected and evaluated for the stable end-product of NO, nitrates, and nitrites, using the Griess reaction according to the Caymanchem instruction manual (Cayman Chemical, USA). We used MTT (Merck, Germany) assay to evaluate macrophage viability after 48-h incubation with different treatments described above.
2.7. Statistical Analysis
GraphPad Prism software version 5.0 (GraphPad Software, USA) was used for statistical analysis of the data. For each group, the mean of three experiments was calculated. Data distribution was analyzed by Kolmogorov–Smirnov test. According to the results of the normality test, Friedman test (non-parametric data) or one-way ANOVA (normally distributed data) for repeated measures, followed by Dunns or Tukey post-test, respectively, were used for statistical comparisons. Data were presented as mean ± SEM. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Immunomodulatory Effects of REB and LEB on Mitogen- Stimulated Splenocytes Proliferation
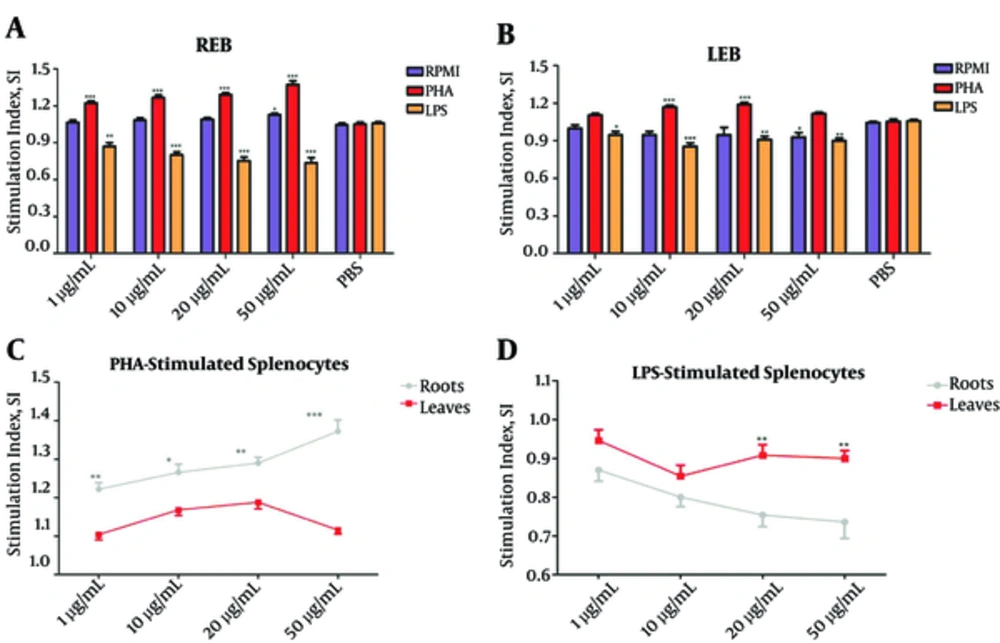
In order to investigate the effects of the REB and LEB on mitogen- stimulated splenocytes proliferation, we evaluated the proliferation of PHA- and LPS-stimulated splenocytes at different concentrations of these extracts, compared to proliferation of PHA- and LPS-stimulated splenocytes without extracts (as PBS group).
We found that REB markedly increase the proliferation of PHA-stimulated splenocytes at all applied concentrations (***P < 0.001; Figure 1A). Similarly, LEB had a stimulatory effect on PHA-stimulated splenocytes proliferation, but only at concentrations 10 and 20 µg/mL (***P < 0.001; Figure 1B). Further analysis revealed that REB is more efficient for increasing PHA-stimulated splenocytes proliferation in comparison with LEB (*P < 0.05; Figure 1C).
Unstimulated splenocytes (RPMI group) and LPS or PHA-stimulated splenocytes were treated with different concentrations of REB or LEB (1, 10, 20, and 50 µg/mL) and cell proliferation was measured by MTT assay. (A) REB significantly increased PHA-stimulated splenocytes proliferation (1 - 50 µg/mL, ***P < 0.001) and decreased LPS-stimulated splenocytes proliferation (1 - 50 µg/mL, **P < 0.01) in comparison with PBS group. (B) LEB significantly increased PHA-stimulated splenocytes proliferation (10 - 20 µg/mL, ***P < 0.001) and decreased LPS-stimulated splenocytes proliferation (1 - 50 µg/mL, **P < 0.01) in comparison with PBS group. (C) REB at all applied concentrations was more efficient for increasing PHA-stimulated splenocytes proliferation in comparison with LEB. (D) REB at high concentrations (20 and 50 µg/mL) was more effective than LEB for suppression of proliferation of LPS-stimulated splenocytes. Stimulation Index (SI): mean OD (optical density) of cells exposed to mitogen/mean OD of cells in media only. *P < 0.05, P < 0.01 and ***P < 0.001. Results are expressed as mean ± SEM of five independent experiments. REB, Roots Extract of Burdock; LEB, Leaves Extract of Burdock.
In contrast to results obtained with PHA-stimulated splenocytes proliferation, both REB and LEB significantly suppress the proliferation of LPS-stimulated splenocytes at all applied concentrations (*P < 0.05; Figure 1A and B). Furthermore, a comparative analysis showed REB at high concentrations (20 and 50 µg/mL) is more effective than LEB for suppression of proliferation of LPS-stimulated splenocytes (**P < 0.01; Figure 1D).
These above data suggested that REB and LEB have immunostimulatory effects on T cells and immunosuppressive effects on B cells; although, REB is more effective than LEB for these properties.
3.2. Altered Production of IL-4 and IFN-γ in PHA-Stimulated-Splenocytes by REB and LEB
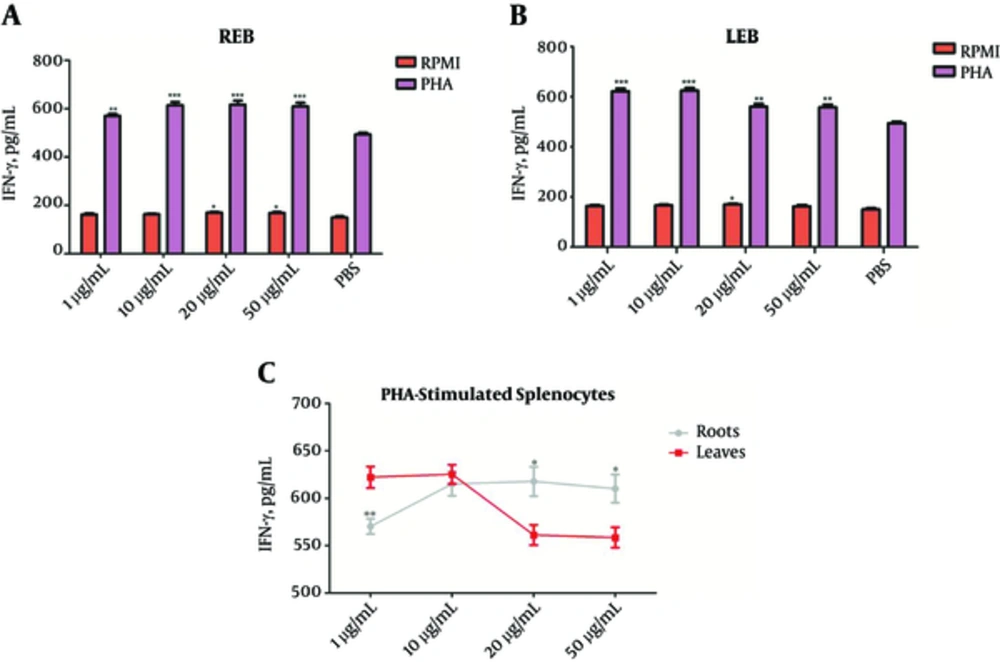
To determination the effects of REB and LEB on production of Th1/Th2 cytokines, we were treated splenocytes and PHA-stimulated splenocytes with different concentrations of REB or LEB and measured IL-4 and IFN-γ levels in cell culture supernatant.
As shown in Figure 2A and B, REB and LEB in concentration ranges of 1 - 50 µg/mL enable to increase IFN-γ production from PHA-stimulated-splenocytes (**P < 0.01), whereas REB at concentrations 20 and 50 µg/mL and LEB only at concentration 20 µg/mL had stimulatory effect on IFN-γ production from splenocytes. Comparative analysis showed that REB at high concentrations (20 and 50 µg/mL) and LEB at low concentration (1 µg/mL) are more effective in stimulating the production of IFN-γ from PHA-stimulated-splenocytes (*P < 0.05; Figure 2C).
The Splenocytes were cultured for 72 hours with or without PHA and treated with different concentrations of REB or LEB. The levels of IFN-γ in the supernatant of cultured splenocytes were measured by sandwich ELISA. (A) REB significantly increased IFN-γ levels in the unstimulated (20 and 50 µg/mL, *P < 0.05) and PHA-stimulated (1 - 50 µg/mL, **P < 0.01) splenocytes. (B) LEB significantly increased IFN-γ levels in the unstimulated (20 µg/mL, *P < 0.05) and PHA-stimulated (1 - 50 µg/mL, **P < 0.01) splenocytes. (C) In comparison between REB and LEB, LEB at low concentration (1 µg/mL) and REB at high concentrations (20 and 50 µg/mL) were more effective in stimulating the production of IFN-γ from PHA-stimulated-splenocytes. *P < 0.05, P < 0.01 and ***P < 0.001. Results are expressed as mean ± SEM of five independent experiments. REB, Roots Extract of Burdock; LEB, Leaves Extract of Burdock.
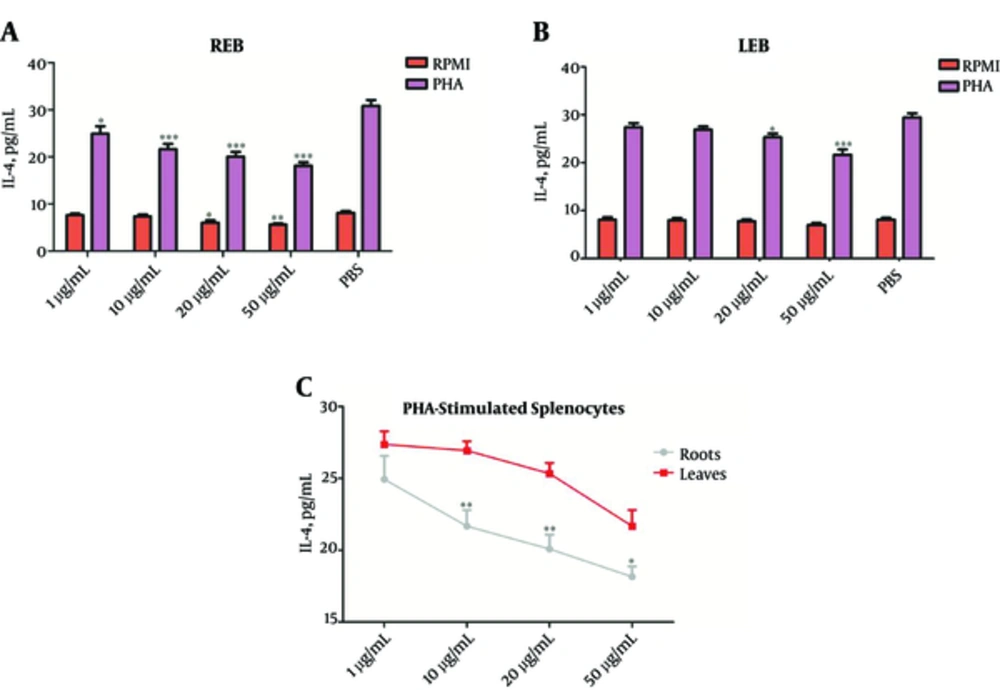
As shown in Figure 3A and B, REB at all applied concentrations and LEB only at high concentrations (20 and 50 µg/mL) significantly suppress IL-4 production from PHA-stimulated-splenocytes (*P < 0.05). Furthermore, our finding showed that only REB at high concentrations (20 and 50 µg/mL) can suppress IL-4 production from splenocytes. In comparison between REB and LEB, it is clear that REB is capable of lower levels of IL-4 secretion from PHA-stimulated-splenocytes than LEB (*P < 0.05; Figure 3C).
The Splenocytes were cultured for 72 hours with or without PHA and treated with different concentrations of REB or LEB. The levels of IL-4 in the supernatant of cultured splenocytes were measured by sandwich ELISA. (A) REB significantly reduced IL-4 levels in unstimulated (20 and 50 µg/mL, *P < 0.05) and PHA-stimulated (1 - 50 µg/mL, *P < 0.05) splenocytes. (B) LEB significantly reduced IL-4 levels in PHA-stimulated splenocytes (20 and 50 µg/mL, *P < 0.05), but not the unstimulated splenocytes. (C) In comparison between REB and LEB, REB at all applied concentrations (except 1 µg/mL) more effective for reduction IL-4 levels in PHA-stimulated splenocytes. *P < 0.05, **P < 0.01 and ***P < 0.001. Results are expressed as mean ± SEM of five independent experiments. REB, Roots Extract of Burdock; LEB, Leaves Extract of Burdock.
Overall, these results indicate that LEB and more strongly REB decrease Th2-associated cytokines production, such as IL-4 and enhance Th1-associated cytokines production, such as IFN-γ in T cells.
3.3. Inhibitory Effects of REB and LEB on NO Production from LPS- Stimulated Macrophages
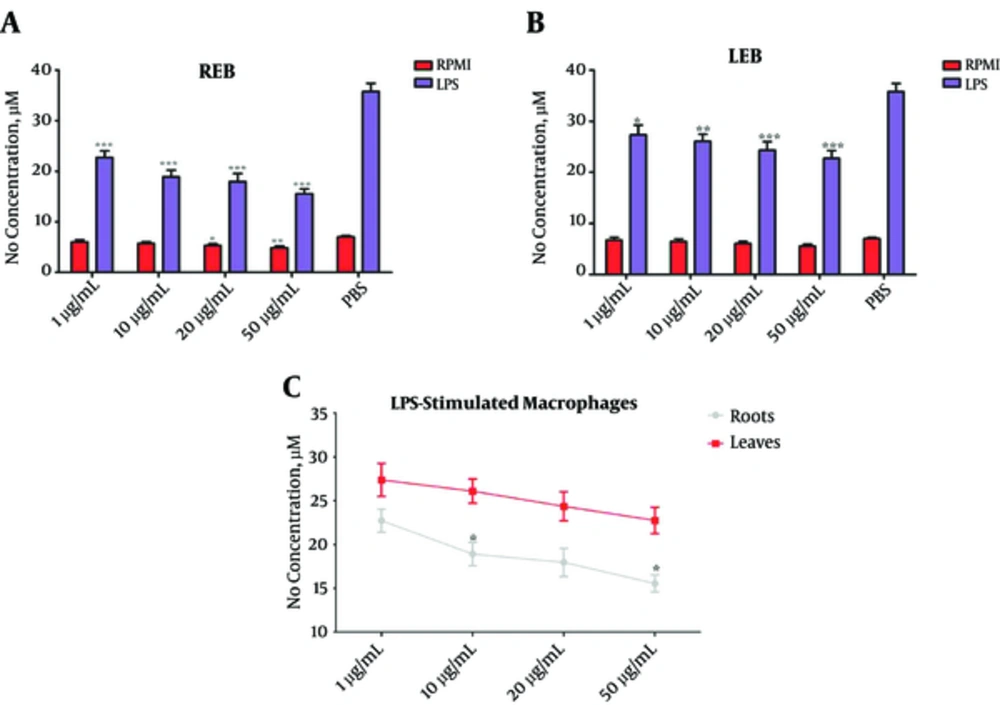
In this study, we treated LPS-stimulated macrophages with different concentrations of REB or LEB and measured NO synthesis by the Griess reaction. Our results showed that both REB and LEB in the concentration range of 1, 10, 20, and 50 µg/mL significantly inhibit NO production from LPS- stimulated macrophages (*P < 0.05; Figure 4A and B). Moreover, in comparison between REB and LEB, REB at concentrations of 10 and 50 µg/mL was more efficient for inhibition of the NO production from LPS- stimulated macrophages (*P < 0.05; Figure 4C). Cell viability test indicated that the inhibitory effects of REB and LEB on NO production from LPS- stimulated macrophages were not due to direct cytotoxicity.
The Macrophages were cultured for 48 hours with or without LPS and treated with different concentrations of REB or LEB. The levels of NO in the supernatant of cultured macrophages were measured by the Griess reaction (A) REB significantly reduced NO levels in unstimulated (20 and 50 µg/mL, *P < 0.05) and LPS-stimulated (1 - 50 µg/mL, ***P < 0.001) macrophages. (B) LEB significantly reduced NO levels in LPS-stimulated macrophages (1 - 50 µg/mL, *P < 0.05), but not the unstimulated macrophages. (C) In comparison between REB and LEB, REB at concentrations 10 and 50 µg/ml was more effective for reduction NO levels in LPS-stimulated macrophages. *P < 0.05, P < 0.01 and ***P < 0.001. Results are expressed as mean ± SEM of five independent experiments. REB: Roots Extract of Burdock, LEB, Leaves Extract of Burdock; NO, Nitric Oxide.
In summary, these results suggest that LEB and more efficiently REB markedly able to control inflammation associated with NO production.
4. Discussion
In traditional medicine, roots and leaves of burdock have been used for different purposes, especially for regulation immune responses and treatment inflammatory disorders. Previous studies showed that various immunomodulatory compounds with different quantities isolated and purified from different tissues of the burdock plant. In present study, we compared the immunomodulatory effects of hydroalcoholic extract of burdock root and leaves. Our results showed that both REB and LEB have suppressive effects on LPS-stimulated splenocytes proliferation (Figure 1A, B), IL-4 secretion from PHA-stimulated splenocytes (Figure 3A, B), and NO production from LPS-stimulated macrophage (Figure 4A, B). The current study also found that both REB and LEB have stimulatory effects on PHA-stimulated splenocytes proliferation (Figure 1A, B), and IFN-γ secretion from PHA-stimulated splenocytes (Figure 2A, B). Although both REB and LEB had similar immunomodulatory effect in vitro, stronger immunomodulatory effect seen in REB.
With regard to the fact that PHA and LPS are mitogens to T lymphocytes and B lymphocytes, respectively, PHA-stimulated splenocytes and LPS-stimulated splenocytes were used for evaluation the effects of REB and LEB on cellular and humoral immune responses. Our results showed that both REB and LEB markedly increase PHA-stimulated splenocytes proliferation and inhibit LPS-stimulated splenocytes proliferation. It is possible, therefore, that LEB and more strongly REB would be able to boost cellular immune responses and attenuate humoral immune responses. These results are in agreement with Matsumoto et al. [17] findings which showed butyrolactone lignans from burdock have antiproliferative activity against B cell hybridoma cell. Tsai et al. [18] showed that arctigenin isolated from burdock suppresses anti-CD3/CD28-stimulated T lymphocyte proliferation. This differs from the findings presented here. A possible explanation for this might be that burdock has many bioactive compounds and arctigeninis only one of them. These similar immunomodulatory properties may support the hypothesis that the quality of bioactive compounds of root and leaves of burdock (such as caffeic acid,Arctigenin, Lappaol F, Diarctigenin, etc.) are similar, but quantity of these bioactive compounds are different.
Th1 and Th2 cells play different roles not only in protection against infections, but also in inflammatory reactions, such as allergic diseases [19]. IL-4 and IFN-γ are the signature cytokines of Th2 and Th1 cells, respectively [19]. We investigated the effects of REB and LEB on IL4 and IFN-γ secretion from PHA stimulated-splenocytes. The results of this study indicate that LEB and more strongly REB increase IFN-γ and suppress IL-4 secretion from PHA stimulated-splenocytes. Due to the central role of cytokines made by Th2 cells (especially IL-4) in allergic reactions, it can thus be suggested that LEB and more strongly REB suppress Th2 cell (but not Th1), and finally control inflammatory disorders associated with th2 such as allergic reactions. In support of this suggestion, Sohn et al. [20] and Knipping et al. (8), indicated that root of burdock has anti-allergic effects. In further support, Sohn et al. also showed that REB suppresses IL-4 secretion from mitogen stimulated splenocytes [20]. On contrary, Sasaki et al. [21] and Rodriguez et al. [22] showed in case report that consumption of root of burdock result in allergic reactions. These conflicting results may be attributed to: 1) differences between in vitro and in vivo studies, (2) differences between raw burdock and boiled burdock, (3) differences between compounds of bioactive secondary metabolites of burdock in different regions.
NO is a free radical that plays an important role in defense against intracellular pathogens and mediate inflammatory tissue damage [23, 24]. It also regulates the functional activity, growth and death of manyimmune and inflammatory cell types [23]. With regard to using burdock for treatment inflammatory diseases, previous studies demonstrated that this treatment effect is related to inhibition NO production from immune cells. In this study, we compared the effect of root and leaves of burdock on NO production from LPS-stimulated macrophages.
Our results showed that both REB and LEB have suppressive effect on NO production, while REB was more effective for these properties. These results are in accord with previous studies indicating that inhibition of inducible nitric oxide synthase (iNOS) expression and NO production are a part of the mechanisms of burdock’s anti-inflammatory action [1]. Wang et al. also demonstrated that REB displays a bioactive action for attenuating excessive NO generation at inflammatory site [7]. Further studies showed that various components, such as Caffeic acid, diarctigenin, arctigenin, Lappaol F, and trachelogeninin root and leaves of burdock are responsible for suppression NO synthesis [11, 25, 26]. HPLC analysis and single quadrupole mass spectrometry of root and leaves of burdock showed that amounts of some of these components in root extract is more than leaves extract [1, 4, 13, 14]. In general, therefore, it seems that root of burdock can be more effective than leaves of burdock for treatment inflammatory diseases related to excessive production of NO.
According to our results we conclude that root of burdock has more efficient than leaves of burdock for 1) suppression of humoral immune responses, 2) shifting Th1/Th2 balance towards Th1 cytokines and 3) inflammatory disorders related to excessive production of NO. These findings suggest that in general root of burdock is better option than leaves of burdock in treating diseases that are caused by immune responses. However, further studies are needed, particularly on comparison of in vivo immunomodulatory effects and mechanisms of action of REB and LEB.