1. Background
In December 2019, cases of pneumonia occurred in the city of Wuhan, Hubei Province, China, due to a new strain of beta-coronavirus. On February 11, 2020, the World Health Organization (WHO) officially named the disease coronavirus disease 2019 (COVID-19) and named the virus causing it the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). The disease spread rapidly throughout China and other countries (2). While the number of confirmed cases of COVID-19 reached about 359 million people worldwide, the Ministry of Health of Iran informed the infection of more than 6,267,000 people and the death of more than 132,200 cases on January 26, 2022, in Iran.
SARS-CoV-2 transmission is mainly through respiratory secretions and close contact between individuals. In terms of time, the highest transmission rate occurs between 2 and 11 days after infection, during which the disease symptoms have not yet appeared, and the person is unaware of his/her infection (3). COVID-19 symptoms are very different at the onset of the disease, and a large number of epidemiological studies have documented SARS-CoV-2 transmission during the incubation period before the onset of symptoms by asymptomatic individuals (4-6). Therefore, diagnosis of mild cases, asymptomatic carriers, and incubation period before the onset of symptoms is important to take effective preventive measures in high-risk situations (5, 7).
Compared to community members, healthcare workers are at higher risk for COVID-19 (8). The risk of infection among healthcare workers is higher, particularly among hospital staff. Depending on where they work, hospital staff are in contact with suspected or confirmed patients and have varying degrees of risk of infection with the virus. If healthcare workers get infected and are not identified, they can transmit the virus to susceptible patients. Due to the high and sometimes high-risk social interactions, investigating the frequency of antibodies against SARS-CoV-2 in high-risk occupations such as medical professions seems necessary for public health policy (9). In fact, serological testing has two advantages; first, it can be used as a tool to identify risk factors for transmission of infection (10, 11), and second, it can be used to assess protective immunity (10).
2. Objectives
The present study aimed to evaluate the seroprevalence of IgG antibodies against SARS-CoV-2 in healthcare workers and to compare the risk of SARS-CoV-2 infection in COVID-19 ward staff with other hospital wards. The results of this study can be used to review health guidelines and how to improve protection in the workplace.
3. Methods
This cross-sectional study was conducted from September 10 to September 20, 2020, at Gerash University of Medical Sciences. Sampling was performed from the clinical, paramedical, and administrative staff of Amir Al-Momenin Hospital and the administrative staff of Gerash University of Medical Sciences willing to participate in the study without specific inclusion or exclusion criteria. A total of 323 people participated in this study. Information regarding demographic characteristics, a history of contact with COVID-19 patients, experience of COVID-19 symptoms, and positive COVID-19 test data were recorded by face-to-face interviews. Amir Al-Momenin Hospital is the main referral center for COVID-19 patients in the city of Gerash. On the other hand, there were administrative staff at Gerash University of Medical Sciences who had no contact with patients with COVID-19. The reason for choosing these two centers was to compare the impact of the workplace on the risk of COVID-19 infection.
3.1. Determination of Serum Levels of Anti-severe Acute Respiratory Syndrome Coronavirus 2 IgG Antibodies
Five mL of venous blood was collected in the test tubes, and after centrifugation, the isolated sera were kept at -20°C. SARS-CoV-2 ELISA kits approved by the Food and Drug Administration (FDA) (Pishtaz Teb, Tehran, Iran) were used to evaluate the presence of SARS-CoV-2 specific IgG antibodies in the samples. According to the manufacturer's instructions, the serum samples were diluted in a ratio of 1:100 and then added to the wells. After incubation and washing and the addition of enzymatic conjugate, the optical density (OD) of each sample (was measured at 450 and 630 nm. To calculate the cut–off point, 0.15 was added to the mean of the negative control ODs, and in the next step, to calculate the cut-off index (COI) of the samples, the sample OD was divided by the cut-off point. According to the manufacturer's instructions, the COI higher than 1.1 was considered positive, and the COI less than 0.9 was considered negative. For COIs in the range of 0.9 - 1.1, the test was repeated with a new blood sample to reach a certain value.
3.2. Statistical Analysis
In this study, statistical analysis was performed using SPSS 20.0 software. Mean and standard deviation were used to present quantitative variables, and classification variables were shown as frequency and percentage. Univariate analysis was performed using the one-way analysis of variance (ANOVA) and non-parametric (chi-square) tests. P-values of P < 0.05 were considered significance levels.
3.3. Ethical Considerations
Written informed consent was obtained from all participants, and voluntary participation was observed in all stages of this study. Ethical considerations in this study included describing how the project was implemented for the participants and protecting privacy in all stages of the study, including interviews, data collection, recording, analysis, and reporting. Sampling was carried out without entering the individuals’ names, and finally, the results of antibody titer for each participant were sent to them by text messages based on their request. This study was approved by the Ethics Committee of Gerash University of Medical Sciences (code: IR.GERUMS.REC.1399.009).
4. Results
Of 323 participants in this study, 233 (72.13%) participants selected from Amir Al-Momenin Hospital were present as a high-risk group, and 90 (27.87%) participants selected from the Faculty of Medical Sciences were present as a low-risk group. The mean age of participants was 38 years (minimum 25 and maximum 55), of which 197 (61%)) were female and 126 (39%) were male. Of 323 participants in this study, 60 (18.6%) had previous polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection. The most common comorbidities among participants included ischemic heart diseases (21.4%), diabetes (21.4%), hypothyroidism (21.4%), hypertension (11.9), and renal diseases (9.5%). Moreover, 21.4% of the patients had more than one comorbidity. Participants’ demographic information is presented in Table 1, and COVID-19 serological results in different groups are shown in Table 2.
| Clinical (n = 130) | Paraclinical (n = 55) | Administrative (n = 138) | Total (n = 323) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 20 (15) | 20 (36) | 86 (62) | 126 (39) |
| Female | 110 (85) | 35 (64) | 52 (38) | 197 (61) |
| COVID-19 symptoms | ||||
| Symptomatic SARS-CoV-2 | 30 (23) | 11 (20) | 30 (22) | 63 (19) |
| Paucisymptomatic SARS-CoV-2 | 37 (28) | 14 (25) | 18 (13) | 69 (21) |
| Asymptomatic SARS-CoV-2 | 63 (48) | 30 (54) | 98 (71) | 191 (59) |
| Symptoms | ||||
| Fever | 34 (26) | 12 (29) | 23 (17) | 69 (52) |
| Cough | 33 (25) | 14 (25) | 24 (17) | 71 (54) |
| Sore throat | 13 (10) | 7 (13) | 10 (7) | 30 (23) |
| Shortness of breath | 10 (8) | 3 (5) | 6 (4) | 19 (14) |
| Headache | 25 (19) | 9 (16) | 15 (11) | 49 (37) |
| Joint pain | 41 (31) | 8 (14) | 26 (19) | 75 (57) |
| Diarrhea | 20 (15) | 6 (11) | 12 (9) | 38 (29) |
| Muscle ache | 26 (20) | 10 (18) | 22 (16) | 58 (44) |
| Fatigue | 53 (41) | 15 (27) | 31 (22) | 99 (75) |
| Nausea | 16 (12) | 1 (2) | 7 (5) | 24 (18) |
| Auditory, olfactory disorder | 13 (10) | 7 (13) | 10 (7) | 30 (23) |
| Patient contact | 126 (97) | 48 (87) | 64 (46) | 238 (73) |
| Underlying diseases b | 23 (55) | 8 (19) | 11 (26) | 42 (13) |
Demographic and Clinical Characteristics a
| Groups | No. | PCR Positive, No. (%) | Statistic (P-Value) | IgG Positive, No. (%) | Statistic (P-Value) |
|---|---|---|---|---|---|
| Clinical | 104 | 16 (18.5) | 3.76 (0.287) | 8 (7.7) | 6.14 (0.105) |
| COVID-19 ward | 26 | 8 (30.76) | 6 (23) | ||
| Paraclinical | 55 | 12 (21.8) | 10 (17.85) | ||
| Administrative | 138 | 24 (17.4) | 20 (14.6) | ||
| Total | 323 | 60 (18.6) | 44 (13.62) | ||
| Asymptomatic SARS-CoV-2 | 191 | 2 (1.05) | 49.29 (0.0001) | 9 (4.71) | 177.82 (0.0001) |
| Paucisymptomatic SARS-CoV-2 | 69 | 10 (14.49) | 10 (14.49) | ||
| Symptomatic SARS-CoV-2 | 63 | 48 (76.2) | 25 (39.68) | ||
| Total | 323 | 60 | 44 |
Seroprevalence of Severe Acute Respiratory Syndrome Coronavirus 2-Specific IgG
Based on the workplace, individuals were divided into three groups: Inpatient wards (n = 130; 40.24%), paraclinical wards (n = 55; 17.02%), and administrative wards (n = 138; 42.72%). It should be noted that 26 (20%) out of 130 people in the inpatient group were employed in the COVID-19 ward subgroup, where COVID-19 patients are hospitalized.
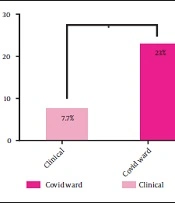
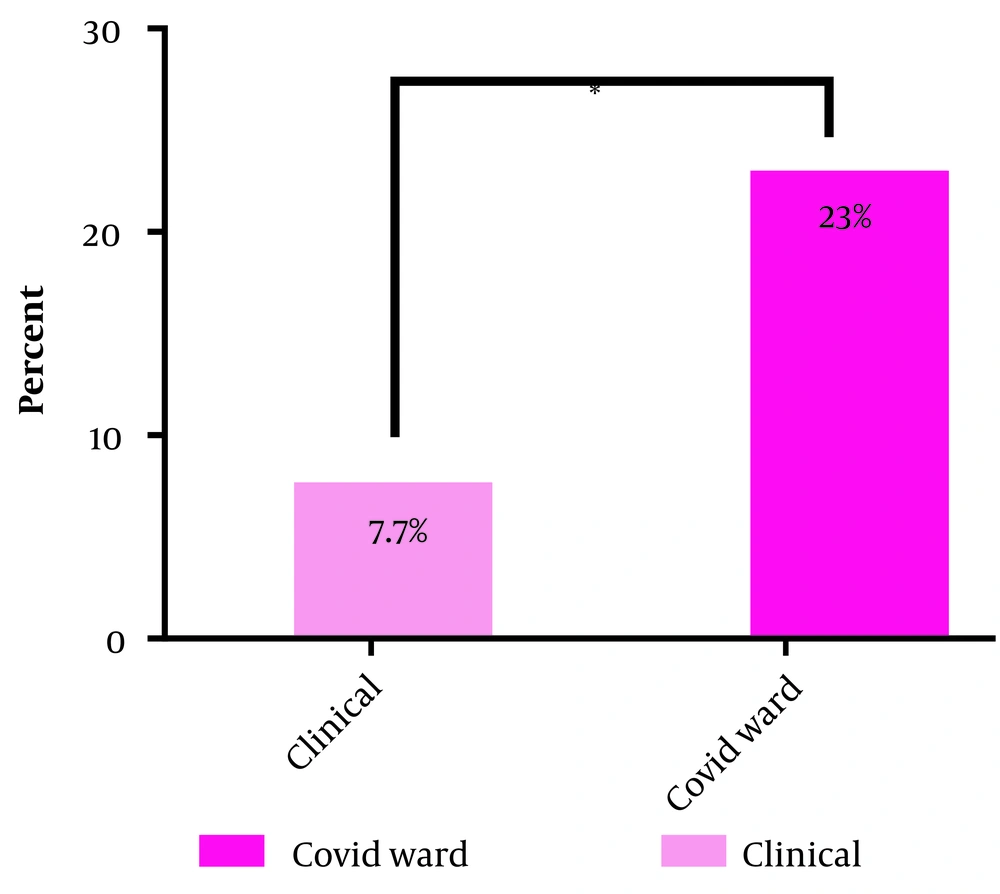
Overall, 44 (13.62%) participants had anti-SARS-CoV-2 IgG antibodies. Of these, 30 (12.9%) hospital staff and 14 (15.55%) faculty staff had coronavirus antibodies. The frequency of individuals with antibodies in different groups is as follows: Fourteen (10.76%) people in the inpatient ward, 10 (17.85%) people in the paraclinical ward, and 20 (14.6%) people in the administrative ward. Examining the mean level of antibodies indicated no significant difference among the three groups. However, in the COVID-19 ward subgroup, 6 (23%) people had anti-SARS-CoV-2 IgG antibodies, which was significantly higher than those working in other wards (P-value: 0.023) (Figure 1).
Based on the number of symptoms, individuals were divided into three groups: The first group included 191 individuals without any history of COVID-19 symptoms (asymptomatic), of which 9 (4.71%) had positive antibodies, and 2 (1.05%) had a history of positive PCR tests; the second group consisted of 69 people who had one to three symptoms (paucisymptomatic), of which, 10 (14.49%) had positive antibodies, and 10 (14.49%) had a history of positive PCR tests; the third category included 63 people with more than three symptoms, of which, 25 (39.68%) had positive antibodies, and 48 (76.2%) had a history of positive PCR tests.
The most common symptoms in antibody-positive subjects were fatigue in 31 (70.45%) people and fever in 25 (56.81%) people, respectively, which perfectly matched with the results of PCR-positive subjects [fatigue in 49 (81.66%) and fever in 40 (66.66%) people].
Of 323 patients, 60 had positive PCR tests, of which antibodies were detectable in the serum of only 31 (51.66%) people. The mean time interval between the onset of symptoms and the sampling time was 63 days (interquartile range [IQR]: 41 - 72).
5. Discussion
It was shown in this study that the prevalence of anti-SARS-CoV-2 antibodies in medical staff was 12.9%, which in similar studies conducted in Iran, such as Bagheri Lankarani et al. (12), Armin et al. (13), and Mortezagholi et al. (14), it was reported to be 5.8%, 29.4%, and 27.8%, respectively. In terms of time, our study was closer to Bagheri Lankarani et al.’s (12) study and was performed after the second wave of the disease in Iran, while the other two studies were performed after the first wave of the disease, about 4 months before our study. However, a lower prevalence of anti-SARS-CoV-2 antibodies was observed in these two studies, indicating better observance of safety principles in the staff under investigation. Comparison of these studies with other studies conducted in the same time frame in other countries shows a higher prevalence of antibodies in studies conducted in Iran; for example, based on Mughal et al.’s study in New Jersey, only 0.83% of subjects had antibodies against SARS- Cov-2 (15). Also, Korth et al.’s study on the German population and Hunter et al.’s study conducted in India revealed that 1.6% of the medical staff had antibodies (16, 17). This difference in the prevalence of anti-SARS-CoV-2 antibodies may be due to the lack of full observance of safety guidelines in medical settings because of the lack of protective equipment compared to other countries.
Comparison of the prevalence of anti-SARS-CoV-2 antibodies in faculty members as a low-risk group (15.5%) and in-hospital staff as a high-risk group (12.9%) were not significantly different, although the faculty staff were shown to be at higher exposure to the virus. This finding is similar to the finding of Hunter et al.’s study conducted on hospital staff in India (17). This slight difference can also be due to the strict observance of health protocols in the hospital environment. Other categories of the investigated subjects were divided into three groups: Administrative, paraclinical, and inpatient. The prevalence of anti-SARS-CoV-2 antibodies was not significantly different in these three groups. This finding is similar to the results of Bagheri Lankarani et al. and Armin et al.’s studies (12, 13); however, in Mortezagholi et al.’s study, the prevalence of antibodies was higher in the medical staff group than in the normal population (14).
In comparison with the inpatient ward subgroups, the frequency of anti-SARS-CoV-2 IgG antibodies was significantly higher in the COVID-19 subgroup than in other inpatient wards, which could be due to long-term contact with COVID-19 patients and higher viral density in the COVID-19 ward (Figure 1).
In the classification based on the number of symptoms, it was observed that with increasing the number of COVID-19 symptoms, the prevalence of anti-SARS-CoV-2 antibodies also increased. This finding was similar to the finding of Poustchi et al.’s (18) study. The study also found that only about 50% of individuals previously diagnosed with COVID-19 by the PCR test had anti-SARS-CoV-2 antibodies in their serum. However, Wajnberg et al.’s study in New York reported an 11% lack of antibody production in individuals with COVID-19 (19). It should be noted that the time interval between the onset of symptoms and sampling in our study was approximately three times longer than that in Wajnberg et al.’s study. This finding could indicate the instability of antibodies produced against SARS-CoV-2 infection.
5.1. Conclusions
The results of this study and similar studies in Iran showed that the rate of virus infection in staff working in medical centers in Iran was significantly higher than in other countries. In particular, the infection rate was much higher in the COVID-19 ward, where the medical staff had been in contact with the COVID-19 patients for a longer period of time. Therefore, it seems necessary to emphasize the more effective use of personal protective equipment and closer monitoring of compliance with health protocols. Also, due to the prevalence of the new Omicron strain, a reminder dose injection is recommended for medical staff.