1. Background
Energy drinks, also called sports drinks, generally refer to beverages containing sugar and various combinations of ingredients purported to "energize" the body and mind. Due to their proposed ergogenic effects, such drinks have become widespread among recreational and elite athletes. Several energy drinks are designed to have optimal carbohydrate (CHO) levels for glycogen replenishment and electrolytes for ion maintenance and prevention of dehydration. They are currently available in the market and are publicized to increase the energy level of the individuals consuming them (1). There were numerous claims of commercially available sports drinks about their benefits for substrate oxidation among athletes (2). In addition, energy drinks (such as sugar-sweetened beverages) are considered significant sources of added sugar in the diet (3) and may be associated with increased prevalence of obesity and overweight, and non-communicable diseases, such as type 2 diabetes and cardiometabolic disease (4, 5). One of the alternative solutions is to use drinks of natural origin.
Grape berries are used as fruits, juice, sultanas, or syrups. There were different grape materials, such as chemicals (carbohydrates, multi-minerals, enzymes, organic acids) and plant chemicals (6). grapes can attenuate free radicals due to their antioxidant components (flavonoids, phenolic acids, anthocyanin, and carotenoids). Anthocyanins (flavonoids) improve health status with anti-inflammatory and antioxidant actions (7). Additionally, anthocyanin increases peripheral blood flow and the amount of oxygen delivered to the muscles during activity through vasodilation and vasorelaxation (8). The synthesis of nitric oxide by endothelial cells and its degradation by free radicals increases in the presence of anthocyanins (9). Grape syrup, like grapes, is rich in minerals (iron, calcium, phosphorus, potassium, and magnesium), sugars (about 66% fast digestible mono-saccharides), and organic acids and phenolic acids (polyphenol and anthocyanin) (10). Grape syrup is a natural nutrient concentrated by adding non-compound additives (6, 10).
The studies on the effect of caffeine, green tea, or New Zealand blackcurrant (compositions rich in polyphenol catechins) have reported increased metabolic rate and oxidation substrate. These studies also have confirmed the role of polyphenols in increasing metabolic rate and oxidation substrate (7, 11, 12). Willems et al. observed that matcha green tea ingestion (1 g day-1) resulted in a lower respiratory exchange ratio (RER) and increased rate of fat catabolism during walking in females (11). Cook et al. also evaluated the effect of New Zealand blackcurrant intake (different doses) on fat catabolism responses during 120 minutes of cycling. They demonstrated that the seven-day intake of New Zealand blackcurrant extract had a dose-dependent effect (emphasizing the 600 mg day-1 dose) on increasing fat oxidation (FO) during prolonged cycling in endurance-trained male cyclists (7).
A high level of carotenoids was observed in white grapes compared to blue-black cultivars. In contrast, blue-black varieties had more total polyphenols (TPC) and higher antioxidant activity (6). Few studies have examined the efficacy of grape-derived beverages during exercise (13-16). For example, de Lima Tavares Toscano et al. investigated the single-dose effects of purple grape juice (10 mL/kg/day) on oxidant and antioxidant enzyme activity, inflammatory indices, physical fitness, and muscular soreness in track and field athletes. These authors reported increased running time to exhaustion and antioxidant enzyme activity (16). In another study, Zolfi et al. evaluated the effect of grape seed extract intake (200 mg day-1) on cardio-metabolic health indices (total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HLD-c), low-density lipoprotein cholesterol (LDL-c), and high-sensitivity C-reactive protein (hs-CRP)) after aerobic exercise (30 minutes with 75% VO2max). Based on the results, there was a significant decrease in TC levels after grape seed extract ingestion (17) Nevertheless, there is no study on the effect of grape syrup drinks on metabolic rate and FO during exercise.
2. Objectives
Therefore, this study examined physiological responses following grape syrup intake at rest, during and after sprint intermittent exercise (SIE) in active students.
3. Methods
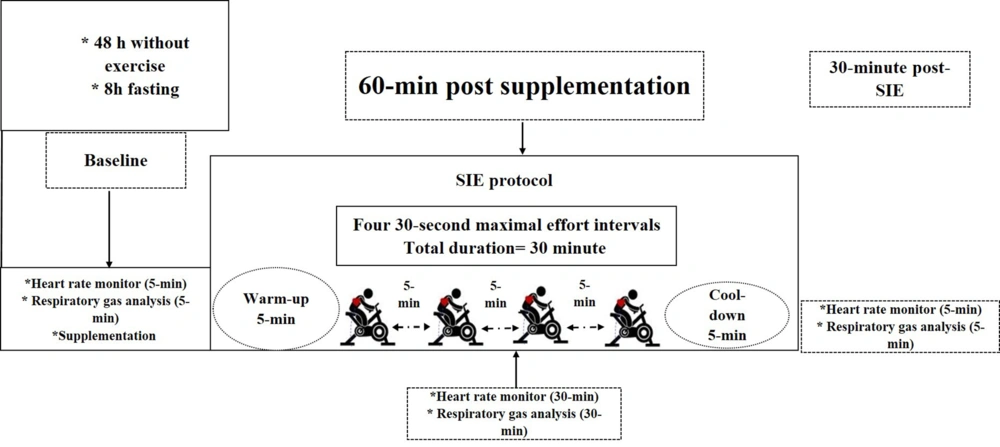
This study was accomplished in a double-blind, crossover design with a placebo. Subjects participated in three sessions (one week apart) in the Sports Physiology Research Laboratory of Birjand University: One familiarization session and two experimental sessions (Figure 1). In the familiarization session, anthropometric characteristics, history of physical activity, and health of the subjects were evaluated (i.e., height, weight, and body mass index (BMI)). Afterward, subjects were introduced to the desired tests and sprint intermittent exercise (SIE) to reduce the effects of learning. Subjects were referred to the laboratory after 8 hours of overnight fasting in both experimental sessions. In addition, subjects were asked to refrain from strenuous exercise for 48 hours before attending the experimental sessions. After entering the laboratory, the subject received a placebo or grape syrup (GS) and rested for 60 minutes. Afterward, rest metabolic measures (using Metamax 3B, Cortex, Leipzig, Germany), and HR (Polar A300; Finland) were recorded for 5 minutes. After the pre-exercise rest period, the subjects began the 30-minute SIE protocol on a computer-integrated cycle ergometer (Monark 894E; Sweden). During SIE, the subjects were measured for exercise Vo2, RER, FO, energy expenditure (EE), and HR (HR). After SIE and 5 minutes of active recovery, subjects rested for 30 minutes, and then post-exercise metabolic measures, such as HR, were measured for 5 minutes (Figure 2).
3.1. Subjects
Twelve active students participated in this study (body mass = 62.77 ± 9.05 kg; height = 173 ± 6.78 cm; BMI = 21 ± 2.5 kg/m2). After completing the physical activity and health history questionnaire, each subject signed a consent form. The subjects met the following inclusion criteria: (1) age = 18 - 32 years, and (2) active physical education students (doing three sessions a week of aerobic or resistance exercise during the last six months). Subjects were excluded from the survey if they reported (1) a medical (cardiovascular, metabolic, pulmonary, renal diseases, high blood pressure, or musculoskeletal disorders) or surgical history in which the physician has forbidden sports activities; and (2) daily use of ergogenic aids or dietary sports supplements 6 weeks prior to the study. Subjects were asked to maintain normal physical activity and exercise levels throughout the three-week intervention period. This study was approved by the Birjand University of Medical Sciences, Iran (IR.BUMS.REC.1397.130).
3.2. Procedures
Anthropometric and metabolic Testing: In the first meeting, the subjects' height (m) and body weight (kg) were measured using a standard stadiometer and a numerical scale, respectively. In the second session, subjects received grape syrup (1.1 mL/ kg of body weight) 60 minutes before implementing the sprint interval exercise (SIE) protocol. In the third session, 60 minutes before the SIE protocol, the subject received the same amount of placebo, similar to grape syrup in terms of smell, taste, sugars, and acids. Before, during, and 30 minutes after the SIE protocol (for 5 minutes), the volume of consumed oxygen (VO2) and produced carbon dioxide (VCO2) of the subjects were measured according to breath to breathe method in liters per minute by Metamax 3B portable ergo-spirometer (Cortex, Germany). Metabolic rate (EE (kcal/hour), oxygen consumption (milliliter/kg.min-1)), substrate oxidation (FO rate (gr/hour), and RER) were calculated through related software. The Metamax 3B portable ergo-spirometer (Cortex, Germany) was used to measure metabolic gas exchanges. The device requires the person to wear a fitted mask to collect the exhaled air. A turbine is attached to the respirator to measure respiratory flow. The feed source and exhaled gas analyzers are worn in a chest harness. The oxygen and carbon dioxide analyzer uses an electrochemical cell and infrared absorptiometry system. Before a subject assessment, the apparatus was calibrated to the known concentration and volume of gases. Calculation of CHO FO rates was assessed according to the non-protein respiratory quotient (R) technique (18, 19):
CHO (gr/hour) = 4.585 VCO2 - 3.2255 VO2
Fat (gr/ hour) = 1.689 (VO2) - 1.701(VCO2)
EE was calculated as follows:
EE (Kcal/hour) = grams of carbohydrates × 4
The proportion of fat used from the respiratory quotient (R = VCO2/VO2) was determined; it is a function of the balance of substrates oxidized by the body. When R is 0.7, 100% of energy is derived from FO, and when R is 1.0, CHO represents 100% of the oxidized fuels. The equations mentioned above were used to calculate the percentage of fat and carbohydrate consumption (19). The gas analyzer device (Metamax3B) has sufficient validity to measure field protocols (20).
3.3. Sprint Intermittent Exercise Protocol
The SIE protocol was performed on a cycle ergometer (Monark 894E; Sweden). In this protocol, the subject performed four repetitions of 30 seconds with maximum effort (Wingate test) and rested for 5 minutes between each repetition. After adjusting the ergometer, the subject started a 5-minute pedal with low-workload intensity (75 watts). Immediately, the subject cycled maximal effort against resistance equal to 7.5% of his body weight for 30 seconds (21). The work intervals mentioned were repeated three more times. After the last interval, the subject cycled with low intensity simultaneously with cool-down (3 minutes at a workload of 30 W). It took a total of 30 minutes to run the SIE protocol (12).
3.4. Supplementation Protocol
Subjects randomly received grape syrup (GS) or a placebo at the desired dose each day of visiting the laboratory in a double-blind manner. Both supplements were liquid and matched for smell, taste, sugars (glucose and fructose), and acids (tartaric and malic). In studies on grape juice, a dose of 5.5 mL/BW has been used (22). As the grape juice is concentrated, the grape syrup is formed in a ratio of 1 to 5 (23). In the present study, grape syrup supplement (Asgari variety) was purchased from traditional gardeners. The modified traditional production method of grape syrup has already been described (24). A Single serving of grape syrup or placebo was 1.1 mL/BW.
3.5. Statistical Analysis
The normality of the dependent variables was checked using the Shapiro-Wilkes test. A two-way (group-time) analysis of variance (ANOVA) for repeated measures was performed to test the significance between groups for EE, VO2, RER, and FO values. Following a significant F value, Bonferroni post hoc test was applied. All statistical analyses were conducted using SPSS version 25. All tests were 2-tailed, and the significance level was P < 0.05. GraphPad Prism 8 software was also used to plot the graphs.
4. Results
The demographic characteristics of the subjects are presented in Table 1. Data regarding variables in different situations (before, during, and after SIE) are shown as mean ± standard deviation in Table 2. In general, there were only between-group differences in RER (P = 0.04). However, the time effect was observed in all variables in different stages (pre, during, and after SIE) (P < 0.05). No time-group interaction effect was also observed in the investigated variables (P > 0.05).
| Subject Characteristics | Age (Y) | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| 22.66 ± 1.65 | 173 ± 6.78 | 62.77 ± 9.05 | 21 ± 2.50 |
Abbreviation: BMI, body mass index.
a Data are expressed as mean ± SD.
| Stage | Grape Syrup | Placebo Group |
|---|---|---|
| Average heart rate (beat/min) | ||
| Pre-SIE | 64.54 ± 3.21 | 68.36 ± 2.45 |
| During SIE | 114.28 ± 2.26 | 115.54 ± 3.80 |
| Post-SIE | 84.50 ± 3.30 | 91.09 ± 4.04 |
| Average VO2 (mL/kg.min) | ||
| Pre-SIE | 6.05 ± 0.26 | 5.63 ± 0.27 |
| During SIE | 16.71 ± 0.60 | 16.09 ± 0.34 |
| Post-SIE | 6.16 ± 0.25 | 5.72 ± 0.33 |
| Average RER | ||
| Pre-SIE | 0.90 ± 0.01 | 0.86 ± 0.01 |
| During SIE | 1.05 ± 0.008 | 1.02 ± 0.11 |
| Post-SIE | 0.75 ± 0.019 | 0.74 ± 0.01 |
| Average fat oxidation (gr/h) | ||
| Pre-SIE | 3.35 ± 0.54 | 4.72 ± 0.38 |
| During SIE | 4.21 ± 0.31 | 4.45 ± 0.41 |
| Post-SIE | 8.70 ± 0.74 | 9.18 ± 0.84 |
| Energy expenditure (Kcal/h) | ||
| Pre-SIE | 113.83 ± 5.14 | 114.45 ± 9.42 |
| During SIE | 287.68 ± 36.69 | 309.63 ± 17.77 |
| Post-SIE | 112.60 ± 6.07 | 111.45 ± 7.40 |
Abbreviations: VO2, oxygen consumption; RER, respiratory exchange ratio. SIE, Sprint intermittent exercise.
a Data are expressed as mean ± SD.
4.1. Energy Expenditure
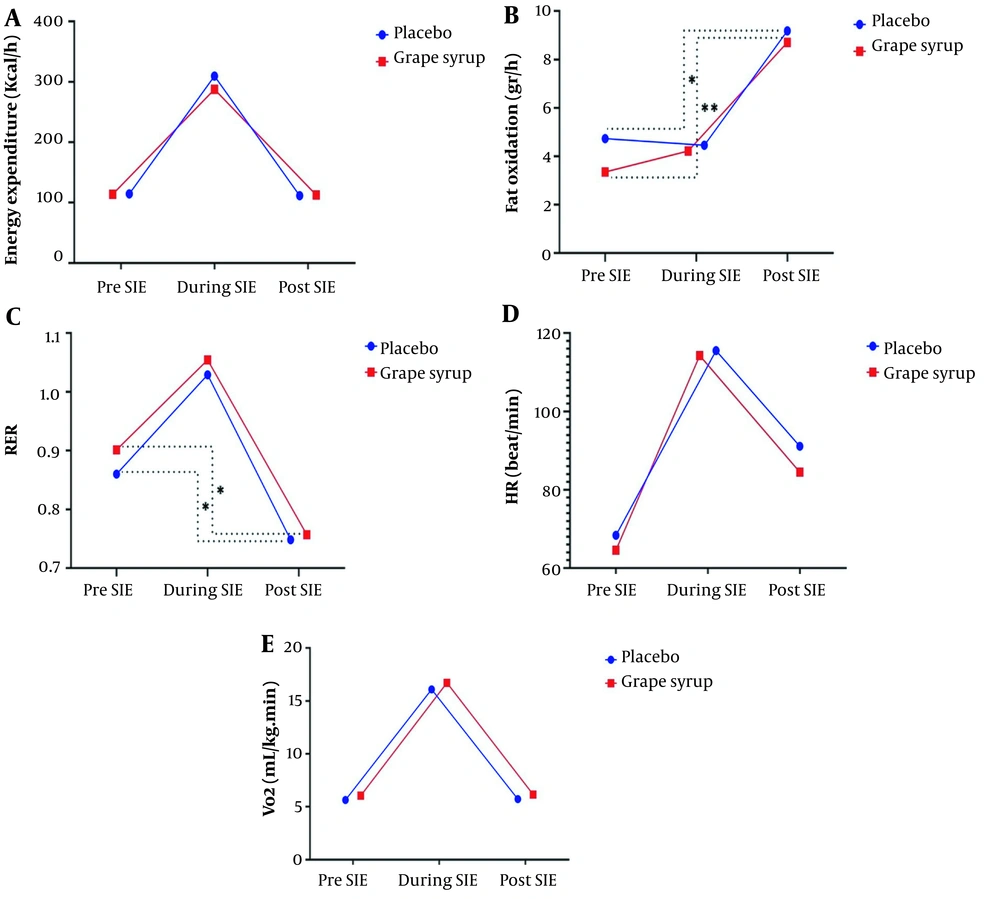
The ANOVA with repeated measures showed no significant difference in EE between groups (P = 0.68). EE in both groups increased during the SIE protocol (P < 0.05) and returned to pre-SIE value (P < 0.05) (Figure 3A).
4.2. Fat Oxidation
The ANOVA with repeated measures showed no significant difference in FO between groups (P = 0.23). FO ratio increased after the SIE compared to pre-SIE (P < 0.05) (Figure 3B).
4.3. Respiratory Exchange Ratio
There was a significant difference in RER between groups (P = 0.04). RER increased during SIE in both situations (P < 0.05) (Figure 3C). In the post-SIE period, RER values in both groups decreased compared to the pre-SIE stage (P < 0.05).
4.4. Heart Rate
The ANOVA with repeated measures showed no significant difference in HR between groups (P = 0.03). HR in both groups increased during the SIE protocol (P > 0.05) (Figure 3D). However, this variable decreased after SIE in both groups (P < 0.05).
4.5. Oxygen Uptake (VO2)
The ANOVA with repeated measures showed no significant difference in VO2 between groups (P = 0.33). The amount of VO2 in both groups increased during the SIE protocol (P > 0.05) (Figure 3F). However, this variable decreased after SIE in grape syrup and placebo situations.
Changes in markers related to metabolic rate and substrate oxidation in response to SIE protocol. (A) energy expenditure; (B) fat oxidation; (C) respiratory exchange ratio (RER); (D) heart rate (HR); (E) oxygen consumption (VO2). Continuous lines represent the change ratio from pre-exercise (pre-SIE) to post-exercise (post-SIE) stages. Dotted lines represent significant changes between exercise stages in the groups or between groups. * Indicates a significant difference from PRE for the respective intervention group (P < 0.05)
5. Discussion
The present study aimed to investigate the effect of grape syrup intake on metabolic rate (VO2 and EE) and substrate oxidation (RER and FO) during and after SIE in active male students. The results showed that the consumption of grape syrup had no significant effect on oxygen uptake. The amount of oxygen uptake increased in both grape syrup and placebo groups during and after the exercise. However, this increase was more significant in the grape syrup group during and after the exercise. Consistent with these results, Wilms et al. investigated the effect of black grapes intake (6 g per day for seven days) during exercise on maximum oxygen consumption. According to their results, there was no significant change in VO2max (25). Although grape syrup has a higher density than grape, the acute intake of grape syrup supplementation has not been sufficient to make a difference in VO2. Because studies on grape syrup are limited, we need to compare our results with those of studies that included a similar supplement with grape syrup (similar components, like carbohydrates, polyphenols, or quercetin). Grapes and their syrups have high polyphenolic and quercetin compounds that affect vascular homeostasis. Each part of a grape, such as fruit, leaves, grape skin, and seeds, has different percentages of polyphenolic compounds. The antioxidant, anti-inflammatory, anti-cancer, anti-microbial, anti-aging effects, and protection of heart tissue are the most critical properties of grape (26). Grape also has regulatory effects on fat metabolism (27). In this regard, Davis et al. showed that the seven days of quercetin supplementation (one of the polyphenol components, 500 mg twice a day) increased VO2max in non-athlete subjects during exercise. This result contradicts our results (28) The reason for this contradiction can be differences in the type of exercise. These authors used aerobic exercise, but we studied the effect of grape syrup during and after SIE. Moreover, the consumption period in their study was longer than ours, which can be effective in increasing VO2. It should be noted that in the present study, the grape syrup intake, in comparison with the placebo, increased after SIE. Although this increase was insignificant, it can benefit weight management strategy because even a slight change in calorie expenditure is essential for people looking for weight management strategies. This increase is significant compared to the placebo, which contains some synthetic carbohydrates (glucose and fructose).
Since the RER value is determined by measuring respiratory gases, it can be used to determine the composition of oxidized foods. For example, if the RER value is one, the cell will use only carbohydrates, and each liter of oxygen consumed produces energy equivalent to 5.5 kcal. While from FO, 4.69 kcal and protein oxidation, 4.46 kcal per liter of oxygen are absorbed. The body naturally uses a combination of different fuels, and the RER values depend on the composition of oxidized food. In the rest situation, the RER value range is from 0.78 to 0.8. Simultaneously with increasing muscle activity, the demand for carbohydrates also increases. As carbohydrate intake increases, the RER value becomes closer to one. Increasing the RER value close to one reflects the body's demand for blood glucose and muscle glycogen. It may also indicate more excretion of carbon dioxide through the blood than carbon dioxide production in the muscles (29). Another result of the present study was the lack of change in the RER of active male students during and after SIE after the intake of grape molasses. RER in both groups increased during exercise compared to pre-exercise. However, these changes were more prominent in the grape syrup group. This is a typical result because RER equal to 1.0 or higher occurs in the SIE, indicating a carbohydrate consumption predominance in the body. In addition, RER decreased in both groups after exercise. However, these changes were more prominent in the grape syrup group. Accordingly, the non-significant increase in RER in the grape syrup group during SIE was compensated by its further decrease in the recovery period. This result is consistent with the results of Cook et al. They investigated the effect of black grape intake (300 mg for seven days) before exercise and showed that RER did not change during exercise (7). The short supplementation period may be one reason for the conclusions in the mentioned studies. Another study result was that the EE of active male students did not change during and after SIE after consuming grape syrup. In other words, the EE of both groups increased during exercise and decreased after exercise. This result is consistent with the results of Cook et al.. They investigated the effect of consuming black grapes for seven days (300, 600, and 900 mg) on substrate oxidation and reported that EE did not significantly change during 120 minutes of cycling exercise. These authors also showed that the level of carbohydrate oxidation during exercise did not change after using different supplement doses (7). Therefore, the dose and period of supplementation are probably the reasons for the ineffectiveness of supplements in the mentioned studies.
According to another result of the present study, grape syrup intake compared to the placebo had no significant effect on the FO of active male students during SIE. However, 30 minutes after exercise, the amount of FO in both groups increased compared to the resting situation, which was significant only in the grape syrup group. In this regard, Cook et al. investigated the effect of grape products or grape polyphenolic compounds on physiological and substrate variables in cyclists, and this supplement (600 mg/day) increased FO (7). In contrast, Cook et al. found that the black grape supplement (300 mg/day) did not change FO during cycling (29). Comparing these two studies, it is clear that higher doses of black grapes might have affected the oxidation of the substrate, possibly due to higher polyphenolic compounds. The same is true in the present study because grape syrup is dense and has more polyphenolic compounds. On the other hand, Zolfi et al. investigated the effect of black grape extract (containing grape polyphenolic compounds) before 30 minutes of aerobic activity on the lipid profile in non-athlete men. The results showed that the black grape extract did not change fat indices and lipid profiles (17). By observing the dose consumed in Zelfi et al.'s study (200 mL) and the present study (1.1 mL per kilogram of body weight; for a 60 kg person = 66 mL), according to the density of grape syrup, its amount can be considered to be around 400 mL. The reason for the ineffectiveness of the supplement in the mentioned study was the low dose of the supplement. Although the duration of aerobic exercise in their study was similar to the present study, the low dose of black grapes and low intensity of aerobic exercise performed compared to the present study caused the supplement's ineffectiveness. In addition, the difference between the present study and those by Cook et al. is related to the exercise duration. Because in these studies, the participants cycled for 120 minutes, while in the present study, regardless of the intensity of exercise, the exercise duration was 30 minutes (7, 29). The physiological reactions that cause fat peroxidation during long-term exercise differ from short-term exercise. For example, fatty acid translocase (FAT/CD36) in the mitochondrial membrane increases after 120 minutes but does not change after 30 minutes (30). In the present study, high exercise intensity was influential in triggering these biochemical reactions. Therefore, to better understand the potential mechanisms involved, it is better to use plasma glycerol in future studies as an indirect marker of lipolysis and free fatty acid during exercise (31) after consuming grape molasses. Accordingly, the dose of grape syrup and the SIE could cause similar effects to that observed in Cook et al.'s study (7).
5.1. Conclusions
In the present study, the grape syrup intake (at a rate of 1.1 mL per kg of body weight) improved metabolic rate and substrate oxidation during the recovery period after SIE, comparable to the placebo containing synthetic carbohydrates. Therefore, it is recommended to use this herbal supplement as a substitute for a carbohydrate drink during SIE. However, more research is needed in this case.