1. Background
The coronavirus disease 2019 (COVID-19) is a new viral disease presenting pneumonia symptoms first reported in Wuhan, China, in 2019. This condition is caused by a new coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Common clinical symptoms of this disease include fever, chills, myalgia, shortness of breath, dry or productive coughs, fatigue, rhinorrhea, sore throat, nausea, diarrhea, and loss of taste and smell (2-5). In the absence of underlying medical conditions, most COVID-19 patients recover without serious complications.
Nevertheless, a small number of patients develop severe complications, such as multiple-organ failure, septic shock, pulmonary edema, severe pneumonia, and adult respiratory distress syndrome (6). According to research, the average age of COVID-19 patients is 56 years, and most affected individuals are men (54%). It is important to note that older individuals who require special care have underlying diseases, such as diabetes, as well as cardiovascular and respiratory diseases (7). Previous studies have revealed that the risk of COVID-19-related death is substantially associated with cardiovascular illnesses, hypertension, congestive heart failure, chronic kidney disease, and cancer (8-10). Most COVID-19 patients experience mild symptoms, such as dry coughs, sore throat, and fever. The currently recommended medications for COVID-19 include antiviral drugs, such as Kaletra (lopinavir/ritonavir), remdesivir, oseltamivir, ribavirin, and sofosbuvir (11-14), immune system modulators, such as chloroquine and hydroxychloroquine (15, 16), and anti-inflammatory drugs, such as corticosteroids (17-19), or a combination of these (20). Vaccination has remained the only solution to the problem since no definitive or particular drug has been demonstrated to be effective against this virus. Therefore, developing effective and reliable therapeutic options for SARS-CoV-2 infection has become a top priority for researchers.
Medicinal plants have been employed since ancient times across the world, but mainly in Asian nations like Iran, India, China, and Japan, as well as in several African nations, due to their accessibility, affordability, and lack of negative side effects. In many developed and developing countries, medicinal plants are currently used as remedies for pain, oxidative stress, cancer, diarrhea, respiratory problems, fever, thrombosis, and infectious diseases (21, 22). Herbal medicines play an important role in improving and balancing immune responses against pathogens and mitigation of hyper-inflammatory states. Therefore, medicinal plants can be widely used to treat SARS-CoV-2 infection and its related complications. Figure 1 displays various possible anti-SARS-CoV-2 therapies (23, 24). Herbal remedies can be used as a complement to conventional medications for COVID-19. Many clinical trials have looked into the applicability of herbal remedies in the fight against SARS-CoV-2. Since many plant-derived medicines have shown antiviral effects, the potential of herbal supplements as therapeutic agents for COVID-19 should not be underestimated. Currently, herbal medicines with antiviral properties are used as adjunctive therapies to confine SARS-CoV-2 propagation, and other herbal treatments are still to be known.
2. Objectives
Considering the critical importance of COVID-19 and the effects of medicinal plants on the reduction of viral symptoms, the current study sought to evaluate the effectiveness of an herbal medicine package (anti-flu Pulmo-Health), along with common medications, in alleviating COVID-19 symptoms in outpatients.
3. Methods
The present study was a double-blinded, randomized controlled trial. The participants in this study were COVID-19 outpatients referring to the Emergency Department of Baqiyatallah Hospital in Tehran in 2020. The subjects who met the inclusion criteria were visited in person; thereafter, they were provided with necessary explanations and requested to sign a written informed consent form. The inclusion criteria comprised a confirmed diagnosis of COVID-19 infection using reverse transcription polymerase chain reaction (RT-PCR) and throat swab specimens or chest CT (computed tomography) scanning, according to the guidelines of the World Health Organization (WHO) (25-27), willingness to participate in the study, the provision of informed consent, and oxygen saturation level less than 94%. Uncontrolled diabetes, gastric bleeding, pregnancy, breastfeeding, immunosuppressive drug use, and chemotherapy in the past month were regarded as exclusion criteria. Also, patients with incomplete documented data and those who withdrew from the trial for any reason were not included in the study.
Based on the latest protocol of the Ministry of Health of Iran at the time of study commencement in 2020, the control group (n = 30) received instructed medicines, such as naproxen 250 mg, methylprednisolone 5 mg, famotidine 20 mg, vitamin C 1000, vitamin D 1000 IU, zinc 15 mg, multivitamins, diphenhydramine (DH), and the placebo (drops) for 10 days. The participants in the intervention group (n = 30) received the herbal package (i.e., a tablespoon of the anti-flu Pulmo-Health syrup every 6 h without drinking water plus 40 drops per day on two occasions; 20 drops dissolved in a glass of water every 12 h and 90 minutes apart from the time of the syrup consumption). The patients in the intervention group also received routine treatment (except for DH) for 10 days. The characteristics of the patients, including age, gender, smoking habits, and marital status, were recorded.
Al-Hawi, Makhzan al- Advieh, and Qanoun Fi-Al-Tibb were initially studied in order to achieve the desired formulation. Thereafter, the knowledge-based Persian traditional medicine database was searched for a list of diseases mimicking COVID-19, as well as information on the medicinal plants used to treat them and how different medications affected viral infection symptoms. After identifying the scientific names of potentially beneficial medicinal plants, their mechanisms of action were studied according to the latest scientific findings and bioinformatics data. Finally, the syrup was prepared based on data from Persian traditional medicine, common drug information, and bioinformatic studies. The syrup contained a variety of plant extracts, including thyme, hyssop, fenugreek, hollyhocks, jujube, malva, licorice, and natural honey, and the drops consisted of the extract of Echinacea purpurea, according to Echinaforce® drops, manufactured by A Vogel Co. (Switzerland). The products were manufactured in a pharmaceutical company under sterile conditions following good manufacturing practice (GMP) regulations. After preparation, they were transferred to tanks and then into final containers. The patients were entitled to take 3–4 tablespoons of the syrup daily, according to the prescribed dosages for each ingredient, and 40 drops of the supplemental drug per day (as noted in the product’s leaflet). The patients were evaluated on days 0, 5, and 10 after study initiation.
The primary outcomes in this study were the oxygen saturation level, respiratory rate, heart rate, frequency of coughs during day and night, shortness of breath score (based on the SOBDA questionnaire), expectoration, and frequency of dry and productive coughs. Moreover, the secondary outcomes included alterations in systolic and diastolic blood pressure, body temperature, headache, nausea, and chills. The shortness of breath with daily activities (SOBDA) questionnaire was applied to assess the impact of daily activities on dyspnea. The items of this 13-item questionnaire are rated on a 6-point scale, ranging from "not at all" to "severe shortness of breath precluding doing daily activities". The validity and reliability of this questionnaire have been confirmed in previous studies (28). Cronbach’s alpha of this tool in the present study was calculated at 94%.
This study was approved by the Medical Ethics Committee of the Baqiyatallah University of Medical Sciences on 20.4.2020 (ID: 156 and code: IR.BMSU.REC.1399.156). Moreover, this study’s protocol was registered in the Iranian Clinical Trial Registration Center (IRCT20160131026298N5). The participants enrolled (n = 60) were randomly assigned into two groups: intervention and control (n = 30 per group), using the random block method (AB, BA) and the Online Random Allocation software. The participants and clinical caregivers were blinded to the group assignments since a placebo was administered to the members of the control group.
3.1. Statistical Analysis and Estimation of Sample Size
The data obtained were analyzed by SPSS software (version 23). In order to describe quantitative variables, mean and standard deviation were used, and frequency and percentage were utilized to present qualitative data. In this study, the t-test, chi-square test, and Fisher's exact test were used to compare the basic variables of the participants between the two groups. Repeated analysis of variance, Cochran’s Q test, and a generalized estimating equation (GEE) logistic regression model were used to investigate within- and between-group variations. The level of significance was set at 5% in all statistical tests. The following formula was used to estimate the sample size (29):
Considering α = 0.05, ß = 0.2%, and d = 0.86, the sample size was calculated at 21 subjects in each group. However, 8 cases per group were considered taking into account 40% sample attrition. Finally, a total of 60 participants were recruited in this study (30 persons per group).
4. Results
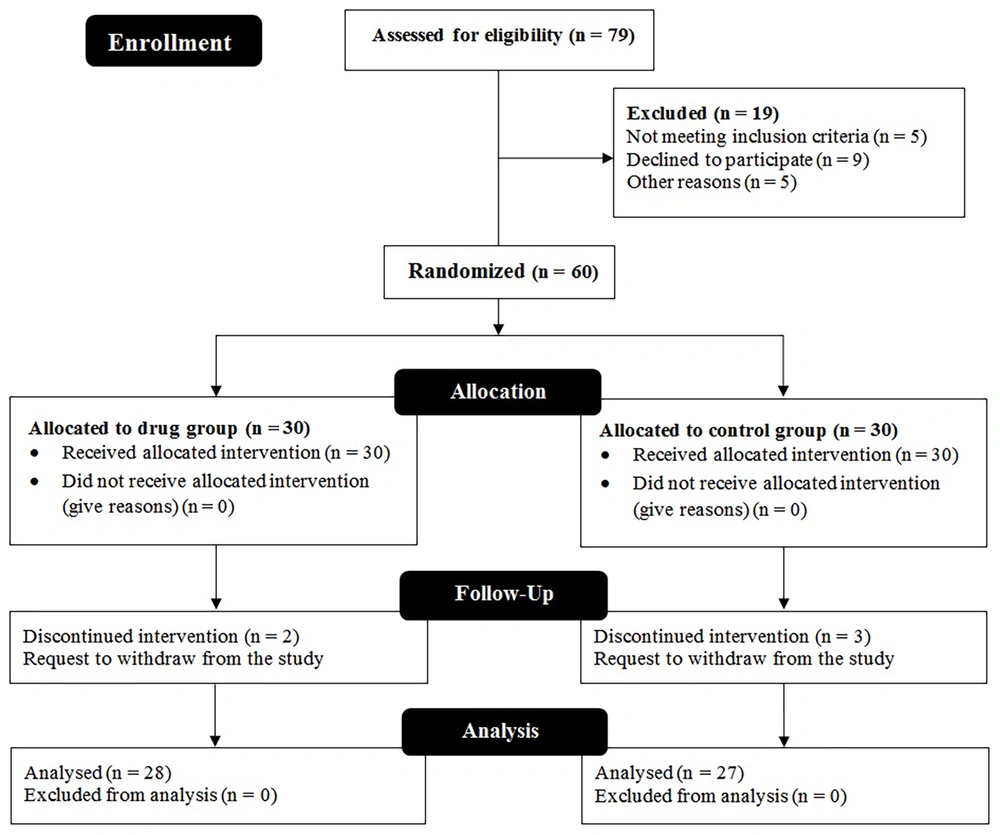
In this study, 76 patients participated in the preliminary assessment, of whom 60 patients were eligible to enter the trial. Finally, the data of 55 participants (27 cases in the control group and 28 subjects in the intervention group) who remained until the end of the study were analyzed. The study’s recruitment and follow-up steps have been schematically illustrated in Figure 2. Table 1 shows the frequency distribution of gender, age, smoking habits, and marital status in the two groups, indicating that most participants were female, married, non-smokers, and within the age range of 31 - 40 years.
| Variables | Group | P-Value | |
|---|---|---|---|
| Routine Treatment + Placebo (n = 27) | Routine Treatment + Pulmo-Health Syrup and Drops (n = 28) | ||
| Gender | 0.53 | ||
| Female | 19 (70) | 17 (61) | |
| Male | 8 (30) | 11 (39) | |
| Age, y | 0.361 | ||
| < 30 | 1 (4) | 1 (4) | |
| 31 - 40 | 12 (44) | 9 (32) | |
| 41 - 50 | 5 (19) | 5 (18) | |
| 51 - 60 | 7 (26) | 5 (18) | |
| > 61 | 2 (7) | 5 (29) | |
| Marital status | 0.758 | ||
| Single | 7 (26) | 6 (21) | |
| Married | 20 (74) | 22 (79) | |
| Smoking status | 0.295 | ||
| Non-smoker | 21 (78) | 25 (89) | |
| Smoker | 6 (22) | 3 (11) | |
Table 2 displays the means and standard deviations of basic variables, such as the respiratory rate, oxygen saturation, body temperature, heart rate, systolic and diastolic blood pressure, the frequency of coughs during day and night, and dyspnea at the beginning of the study. Table 3 presents the frequency distribution of the patients’ symptoms, including smell and taste problems, diarrhea, insomnia, anorexia, shortness of breath, nausea, fatigue, headache, dry and productive coughs, sore throat, and chills at the beginning of the study in the two groups. The data presented in Tables 1 -3 denote the homogeneity of the participants in terms of basic demographic and clinical variables.
| Variables | Groups | P-Value | |
|---|---|---|---|
| Routine Treatment + Placebo | Routine Treatment + Pulmo-Health Syrup and Drops | ||
| BMI | 26.77 ± 4.45 | 25.28 ± 3.3 | 0.35 |
| Respiratory rate | 23.22 ± 7.1 | 23.89 ± 2.56 | 0.281 |
| Oxygen saturation | 90.96 ± 1.37 | 90.18 ± 1.36 | 0.31 |
| Body temperature | 38.71 ± 0.45 | 38.85 ± 0.3 | 0.775 |
| Heart rate | 107.74 ± 4.13 | 108.61 ± 4.93 | 0.51 |
| Systolic blood pressure | 13.42 ± 1.38 | 13.96 ± 0.7 | 0.157 |
| Diastolic blood pressure | 9.51 ± 1.38 | 9.11 ± 0.76 | 0.76 |
| Daytime cough score | 2.07 ± 0.86 | 2.29 ± 0.66 | 0.24 |
| Nighttime cough score | 2.41 ± 0.75 | 2.46 ± 0.51 | 0.932 |
| Shortness of breath score | 45.59 ± 4.81 | 46.36 ± 7.41 | 0.839 |
a Values are expressed as mean ± SD.
| Symptoms | Groups | P-Value | |
|---|---|---|---|
| 1 (n = 27) | 2 (n = 28) | ||
| Smell problems | 0 (0) | 1 (4) | 0.999 |
| Taste problems | 0 (0) | 1 (4) | 0.000 |
| Diarrhea | 5 (19) | 3 (11) | 0.469 |
| Insomnia | 22 (81) | 27 (96) | 0.101 |
| Anorexia | 19 (73) | 25 (89) | 0.169 |
| Shortness of breath | 24 (89) | 28 (100) | 0.111 |
| Nausea | 5 (19) | 14 (50) | 0.23 |
| Fatigue | 26 (96) | 28 (100) | 0.491 |
| Headache | 24 (89) | 28 (100) | 0.111 |
| Dry cough | 27 (100) | 28 (100) | 0.999 |
| Productive cough | 1 (4) | 0 (0) | 0.999 |
| Sore throat | 26 (96) | 28 (100) | 0.999 |
| Chills | 19 (70) | 27 (96) | 0.12 |
a Values are expressed as No. (%).
b Group 1: Routine treatment + Placebo; Group 2: Routine treatment+ Pulmo-Health syrup and drops.
4.1. Assessment of Outcomes
Fifty-five patients completed the study, of whom 65% were female. The clinical symptoms of COVID-19 were compared between the patients in the two groups on days 5 and 10 (Table 4). The means and standard deviations of these symptoms were calculated during the treatment period for all patients, except for cough intensity, which was assessed on a qualitative scale due to the lack of a reliable cough frequency assessment tool and patients’ non-compliance. The patients were interviewed regarding these measures, and improvement rates were compared before (day 0) and after (days 5 and 10) the intervention.
| Variables and Groups | Baseline (Day 0) | Day 5 | Day 10 | P-Value | |
|---|---|---|---|---|---|
| Within-Group | Between-Group | ||||
| Respiratory rate | < 0.001 | ||||
| 1 | 23.22 ± 7.10 | 24.85 ± 1.83 | 22.48 ± 1.37 | 0.120 | |
| 2 | 23.89 ± 2.56 | 19.89 ± 2.27 | 15.29 ± 2.76 | < 0.001 | |
| Oxygen saturation | 0.013 | ||||
| 1 | 90.96 ± 1.37 | 91.67 ± 1.44 | 92.23 ± 10.66 | 0.770 | |
| 2 | 90.18 ± 1.36 | 93.68 ± 0.82 | 96.4 ± 0.58 | < 0.001 | |
| Heart rate | < 0.001 | ||||
| 1 | 107.74 ± 4.13 | 105.44 ± 4.96 | 95.33 ± 5.28 | < 0.001 | |
| 2 | 108.61 ± 4.93 | 98.89 ± 5.36 | 89.4 ± 5.18 | < 0.001 | |
| Severity of nighttime coughs | < 0.001 | ||||
| 1 | 2.41 ± 0.75 | 2.40 ± 0.58 | 1.74 ± 0.66 | < 0.001 | |
| 2 | 2.46 ± 0.51 | 1.04 ± 0.43 | 0.18 ± 0.39 | < 0.001 | |
| Severity of daytime coughs | < 0.001 | ||||
| 1 | 2.70 ± 0.68 | 1.85 ± 0.60 | 1.41 ± 0.75 | 0.003 | |
| 2 | 2.29 ± 0.66 | 0.46 ± 0.64 | 0.001 ± 0.001 | < 0.001 | |
| Systolic blood pressure | 0.531 | ||||
| 1 | 13.42 ± 1.38 | 13.43 ± 0.49 | 12.97 ± 0.53 | < 0.001 | |
| 2 | 13.96 ± 0.70 | 13.5 ± 0.47 | 12.49 ± 0.43 | < 0.001 | |
| Diastolic blood pressure | < 0.001 | ||||
| 1 | 9.51 ± 1.38 | 9.19 ± 0.58 | 8.55 ± 0.64 | < 0.001 | |
| 2 | 9.11 ± 0.76 | 8.65 ± 0.38 | 8.30 ± 0.32 | < 0.001 | |
| Body temperature | < 0.001 | ||||
| 1 | 38.71 ± 0.45 | 38.71 ± 0.62 | 37.89 ± 0.31 | < 0.001 | |
| 2 | 38.85 ± 0.30 | 38.30 ± 0.19 | 37.41 ± 0.16 | < 0.001 | |
| Shortness of breath score | < 0.001 | ||||
| 1 | 45.59 ± 4.81 | 34.22 ± 4.66 | 26.07 ± 5.72 | < 0.001 | |
| 2 | 46.36 ± 7.41 | 28.64 ± 6.60 | 13.90 ± 3.77 | < 0.001 | |
a Values are expressed as mean ± SD.
b Group 1: Routine treatment+ Placebo; Group 2: Routine treatment+ Pulmo-Health syrup and drops.
Based on the results of this study, COVID-19 patients in the intervention group, who were administered routine medications in combination with the herbal syrup and drops, demonstrated better therapeutic performance in terms of the respiratory rate, oxygen saturation level, expectoration, the frequency of dry and productive coughs, frequency of coughs, body temperature, and shortness of breath as compared to the control group. These results could be explained by the shorter recovery time in the intervention group than in the control group. Taste and smell conditions were examined on days 5th and 10th of the study, showing no significant difference in the restoration of these senses between the two groups, indicating that the studied herbal package had no beneficial effect on these symptoms.
Oxygen saturation increased significantly in the intervention group compared to the control group five and ten days after starting the consumption of the herbal medicine and reached 96% on the 10th day, reflecting an increase in the tissue oxygenation level in the intervention group (P < 0.001), leading to a reduction in the use of oxygen capsules in the intervention group compared to those who received standard care alone.
As depicted in Table 4, the respiratory rate was still >20 breaths per minute on the fifth day after starting the experiment in both the intervention and control groups, showing no significant difference. Nonetheless, the respiratory rate returned to normal in the intervention group on day 10th, showing a significant improvement compared to the control group (P < 0.001). Shortness of breath and the frequency of coughs during day and night were compared between the two groups on the 5th and 10th days of treatment, reflecting a significant decrease in both in the intervention group compared to the control group (P < 0.001). The severity of day and nighttime coughs showed marked improvement in the intervention group compared to the control group on days 5th and 10th of treatment (P < 0.001). Dry and productive coughs significantly decreased in the intervention group five days after starting drug administration (P < 0.001).
The frequencies of dry cough, productive cough, shortness of breath, and expectoration during the follow-up have been shown in Table 5. As shown, marked improvements were observed in respiratory symptoms in both groups on days 5th and 10th. The reduction rate in dry coughs was almost twice in the intervention group compared to the control group. Moreover, other clinical symptoms, such as expectoration, lung cleansing, and dyspnea, showed significantly higher rates of improvement in the intervention group compared to the control group (P < 0.001). On the fifth day of the study, dry coughs disappeared while productive coughs persisted in all patients in the intervention group, indicating the considerable anti-inflammatory and expectorant effects of this herbal medicine. On the other hand, dry coughs persisted in the patients of the control group without any sign of expectoration on the fifth day (P > 0.05). However, based on the patients’ announcements in the control group, a marked increase was observed in the amount of sputum produced on days 8th and 9th, while on these days, sputum production significantly decreased in the patients of the intervention group. Regarding the amount of sputum production on the fifth day, a significant increase was detected in the intervention group compared to the control group, while this parameter showed a marked reduction on day 10th. As previously mentioned, dry coughs completely resolved in the intervention group on day fifth (in parallel with a sharp rise in productive coughs at this time), and the patients remained free of dry coughs until the end of the study (i.e., day 10th).
| Variables and Groups | Day 0, % | Day 5, % | P-Value (0 - 5 Days) | Day 10, % | P-Value (0 - 10 Days) | ||
|---|---|---|---|---|---|---|---|
| Within-Group | Between-Group | Within-Group | Between-Group | ||||
| Dry cough | < 0.001 | < 0.001 | |||||
| 1 | 100 | 96 | 0.301 | 85 | 0.040 | ||
| 2 | 100 | 0 | < 0.001 | 0 | < 0.001 | ||
| Productive cough | < 0.001 | < 0.001 | |||||
| 1 | 4 | 8 | 0.255 | 41 | 0.007 | ||
| 2 | 0 | 100 | < 0.001 | 18 | < 0.001 | ||
| Shortness of breath | < 0.001 | < 0.001 | |||||
| 1 | 89 | 100 | 0.080 | 93 | 0.319 | ||
| 2 | 100 | 36 | < 0.001 | 0 | < 0.001 | ||
| Expectoration | < 0.001 | < 0.001 | |||||
| 1 | 0 | 4 | 0.212 | 48 | < 0.001 | ||
| 2 | 0 | 100 | < 0.001 | 36 | < 0.001 | ||
a Group 1: Routine treatment+ Placebo; Group 2: Routine treatment+ Pulmo-Health Syrup and drops
4.2. Follow-Up
The patients responded differently to the treatments depending on the disease stage and the impact of the virus on their digestive or respiratory systems. According to previous studies, most COVID-19 patients generally return to the doctor one or two months after recovery, complaining of persistent coughs and dyspnea. In the current trial, 12 individuals from the control group returned as they had persistent cough and dyspnea, while there were no complaints of cough or dyspnea in the intervention group during follow-up visits, indicating that the herbal medication package performed better than the standard treatment in relieving shortness of breath, cough, and expectoration in COVID-19 patients.
5. Discussion
Since the outbreak of COVID-19, the use of various traditional herbal medicines, alone or in combination with other medications, has yielded promising therapeutic results and outcomes in infected patients (30). Various herbal products may directly hinder the reproduction or entrance of the SARS-CoV-2 virus (31), suggesting them as suitable alternative agents that can be used in combination with other conventional medications to treat or even prevent the COVID-19 infection (32).
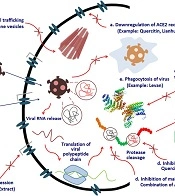
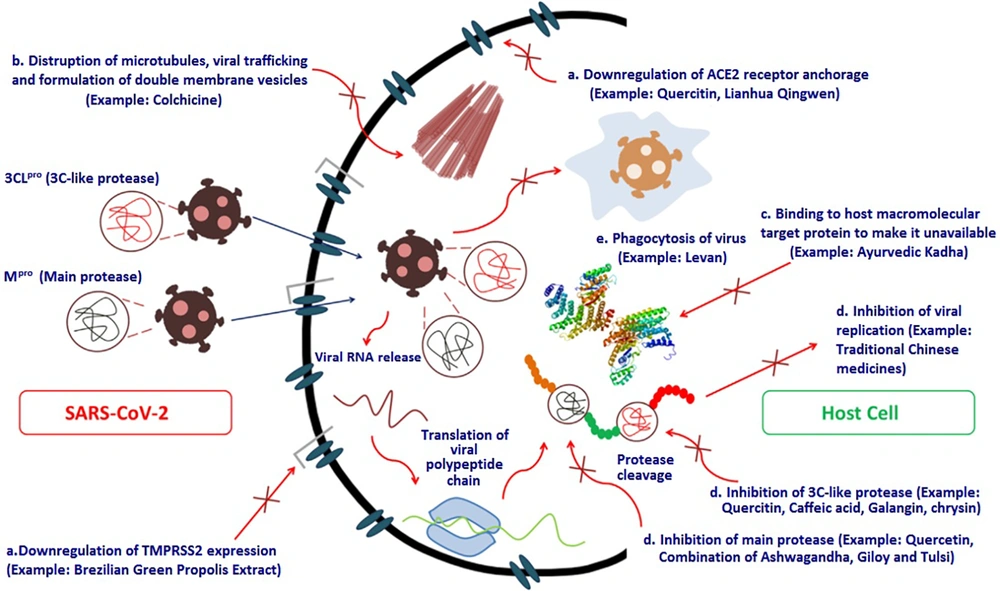
In this study, various medicinal plants that were assumed to be potentially helpful in treating the SARS-CoV-2 infection were amassed to produce the Pulmo-Health herbal syrup. Bioinformatic tools have been used comprehensively to assess the immunomodulatory effects of herbal compounds and the inhibitory effects of certain plant-derived peptides on the spike protein of SARS-CoV-2 (33). Figure 3 depicts the various routes through which secondary metabolites, such as potent herbal chemicals, affect the function of essential coronavirus proteins (34).
The present study aimed to assess the efficacy of an herbal medicine package, anti-flu Pulmo-Health, in alleviating the symptoms of COVID-19 outpatients. According to the findings of the present study, COVID-19 patients in the intervention group, who received the herbal syrup and drops in addition to the routine treatment, demonstrated better therapeutic performance in terms of the respiratory rate index, oxygen saturation level, expectoration, frequency of dry and productive coughs, frequency of day and nighttime coughs, fever, and shortness of breath as compared to their counterparts in the control group who received only routine treatments.
One of the explanations for the potent anti-COVID-19 effects of the Pulmo-Health herbal syrup may be the immunomodulatory functions of its ingredients. The secondary metabolites of medicinal plants, such as alkaloids, flavonoids, polyphenols, tannins, lignins, coumarins, and terpenoids, are assumed to be effective in the treatment of infections caused by pathogenic microorganisms due to their ability to suppress viral proteins and enzymes and prevent viral penetration into and replication in host cells. Accordingly, numerous potential mechanisms have been suggested for the antiviral effects of plant-derived substances, some of which are depicted in Figure 3. These findings have raised hopes for developing effective herbal anti-coronavirus drugs.
According to the results of this study, it could be argued that the herbal compounds existing in the Pulmo-Health syrup and drops improved the immune system defense against the viral disease through mechanisms like enhancing the production of cytokines by monocytes, inducing the production of interferons, boosting the activity of phagocytes, enhancing intestinal probiotic flora, inducing immunoglobulin production by lymphocytes, and neutralizing hydrogen peroxide and oxygen free radicals. Collectively, these effects can trigger the destruction of viral particles. Moreover, the compounds in the syrup could reduce mucus viscosity and stimulate pulmonary secretions by improving the function of respiratory cilia. It is important to note that these secretions, along with the antimicrobial properties of the syrup’s constituents, can prevent secondary bacterial infections.
This study had some limitations, including small sample size and not assessing and controlling the patients’ psychological conditions. On the other hand, this study represented a well-designed trial (double-blinded, randomized, placebo-controlled) with a high homogeneity between the control and intervention groups. Also, the patients were followed up after recovery, which is another strength of this study.
5.1. Conclusions
This double-blinded randomized controlled trial demonstrated that the studied herbal package (i.e., the Pulmo-Health syrup and supplementary drops), together with conventional medications, significantly improved the respiratory symptoms of COVID-19 patients, such as cough, shortness of breath, and expectoration. Also, it was found that the combination of this herbal medicine with standard medications could markedly shorten the duration of COVID-19 treatment. The main biological characteristics of this product seemed to include anti-inflammatory, expectorant, antitussive, immune-enhancing, and anti-SARS-CoV-2 effects. The results showed that the substitution of the herbal syrup and drops for diphenhydramine yielded acceptable outcomes, confirming the beneficial effects of this herbal medicine, along with routine medications, on the respiratory symptoms of COVID-19 patients.