1. Background
As a progressive chronic metabolic impairment, diabetes is one the most common non-communicable diseases and also one of the top ten causes of death in the world (1). Diabetes greatly increases the risk of developing other diseases, especially cardiovascular problems (2, 3). It has been reported that cardiovascular diseases (CVDs) are three times more prevalent among diabetic people than among their healthy counterparts (4). Indeed, CVDs are among the leading causes of death in diabetic patients (5). There are reports showing that about 65% of deaths among people with type 2 diabetes (T2D) can be attributed to cardiovascular problems. Diabetic people also carry several times higher risks of heart failure, stroke, and death due to coronary artery disease than non-diabetic people (6, 7). Some studies have shown a link between hyperglycemia, increased oxidative stress, and diabetes complications (8). It has been suggested that one of the mechanisms by which diabetes damages the myocardium is the reduction of mitochondrial biogenesis capacity.
According to several studies, diabetes significantly reduces mitochondrial biogenesis capacity in cardiac cells. A variety of signaling pathways, including protein kinase B (PKB/AKT) and adrenergic receptor-derived routes, are believed to play a role in the regulation of mitochondrial biogenesis by increasing cardiac muscle stretching (9). A balanced mitochondrial biogenesis capacity is decisive for appropriate cardiac compatibility, and any outage of the pathways involved in this process can have irreversible consequences for the heart (10). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is the main regulator of mitochondrial biogenesis by acting as a cellular receptor, facilitating the release of mitochondrial proteins (8). This process may also be affected by Sirtuin 1 (SIRT1), a member of the family of deacetylase proteins with a significant role in reducing oxidative stress and controlling homeostasis (11). Depending on how they deacetylate transcription factors such as PGC-1α, sirtuins are involved in a wide variety of vital functions, including the control of free radical production, lipid oxidation, and mitochondrial biogenesis (12, 13).
The most common and effective interventions for controlling diabetes are lifestyle modifications and anti-diabetic medications. While anti-diabetic medications like beta blockers and insulin are widely used by many patients, there are also many reports of medication non-adherence (14) and drug interactions (15), as well as treatment failure and contradictory therapeutic outcomes (16). In comparison, exercise is a safe and effective intervention for controlling diabetes, obviating many of the above-mentioned problems. Several studies have investigated the effects of exercise on the expression of PGC-1α and SIRT1 (17, 18). In one of these studies, increased PGC-1α gene expression was observed immediately after acute endurance exercise, but the exercise did not change SIRT1 expression (19). In another study, long-term endurance exercise at both low and high intensities increased SIRT1 expression; however, PGC-1α expression was increased only after high-intensity exercise (20). In a study by Gurd et al., 6 weeks of high-intensity interval training increased the level of PGC-1α protein, the gene expression of antioxidant enzymes (such as catalase), and SIRT1 activity, where PGC-1α elevation was attributed to nuclear SIRT1 activity (21).
Herbal medicines are also a popular means for controlling diabetes in people who worry about the potential side effects of chemical drugs (22, 23). Berberine (BBR) is an alkaloid found in barberry (Berberis vulgaris) and possesses potentially strong hypoglycemic, anti-inflammatory, analgesic, and antioxidant effects (24). In one study, administering this substance at doses of 50 and 100 mg/kg of body weight had significant impacts on insulin resistance in rats with streptozotocin-induced diabetes (25). However, despite the positive effects of this supplement on diabetes, it can have side effects such as nausea, vomiting, diarrhea, and miscarriage (24). Thus, further studies are needed to determine whether its adjusted doses can provide favorable therapeutic outcomes without causing side effects.
2. Objectives
Considering that both exercise and BBR supplementation can potentially ameliorate diabetes and its associated cardiomyopathy, this study examined the effect of eight weeks of aerobic exercise combined with BBR supplementation on the expression of the SIRT1 and PGC-1α genes in the cardiac muscle of diabetic male Wistar rats.
3. Methods
This research was an experimental animal study. In all stages of the study, the requirements for managing and treating laboratory animals as per CONSORT guidelines on animal research were observed (26). All stages of the research were also confirmed by the Research Ethics Committee of the Najafabad Islamic Azad University (ethics code: IR.IAU.NAJAFABAD.REC.1400.061). The experiments were conducted on 56 male Wistar rats (2 weeks old, weighing 278.26 ± 18.06 grams). The rats were prepared from the Iran Pasteur Institute and maintained in 8 fiberglass cages (1 × 1 × 1 m) located in a room with controlled conditions (12: 12 light-dark cycles). The animals had free access to water and were fed standard rat food containing 3.5 - 4.5% crude fat, 23% protein, 4 - 4.5% crude fiber, and adequate minerals and vitamins (Behparvor Co., Iran) (26). In the familiarization phase, the rats were managed to walk on a treadmill at a speed of 4 - 5 m/s (5 - 10 minutes a day, five days a week) for two weeks so that they could adapt to the treadmill and laboratory environment.
3.1. Grouping
Fifty-six adult male Wistar rats were allocated into seven groups of eight animals. First, eight of the rats were randomly chosen to serve as healthy control animals (C). The remaining rats were then randomly divided into six groups: diabetic control group (D), diabetic + 15 mg/kg of berberine chloride (D-B1), diabetic + 30 mg/kg of berberine chloride (D-B2), diabetic + aerobic exercise (DT), diabetic + aerobic exercise + 15 mg/kg of berberine chloride (DT-B1), and diabetic + aerobic exercise + 30 mg/kg of berberine chloride (DT-B2).
3.2. Type-2 Diabetes Induction
Diabetes was induced by the intraperitoneal (IP) injection of streptozotocin (manufactured by Sigma, product code: S0130) at a dose of 60 mg/kg in 0.1 M citrate buffer, PH = 4.5 (27). All animals in the healthy control group were injected with an equal volume of the normal saline solution instead of streptozotocin to reproduce the stress of injection. For 48 hours after streptozotocin injection, the rats were given a 5% glucose solution instead of water to prevent mortality. At 72 hours after streptozotocin injection, blood glucose concentration was measured after 12 hours of overnight fasting using a Glucocard-01 glucometer (Japan), for which a small wound was created by a lancet in the tail (28). Rats with blood sugar higher than 300 mg/dL were included in the study as diabetic animals. The day on which blood sugar was measured was designated as day zero.
3.3. Exercise Protocol
Exercise was planned at the intensity of 50 - 55% of maximal oxygen consumption (VO2 max). The animals in the exercise intervention groups were managed to run on a treadmill three times a week for eight weeks. According to Chae et al. (29), the exercise protocol consisted of running at gradually increasing speeds and gradually increasing durations, along with 3 minutes of warming up at the beginning of each session and cooling down at a rate of 4-5 m/min at the end of each session. The rats were not given electric shocks on the treadmill to avoid causing extra stress during the exercise. The details of the exercise protocol are provided in Table 1.
| Exercise Variables | Week | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | |
| Speed (m/min) | 4 - 5 | 4 - 5 | 10 | 10 | 14 - 15 | 14 - 15 | 17 - 18 | 17 - 18 |
| Duration (min) | 5 | 5 | 10 | 20 | 20 | 30 | 30 | 40 |
3.4. Berberine Chloride Supplementation
The supplement used in this study was 90% pure berberine chloride hydrate powder manufactured by Sigma (product code: 14050) extracted from the barberry plant. The appropriate amount of the powder was dissolved in normal saline solution, and the product was fed orally (gavage) to animals at doses of 15 and 30 mg/kg (milligrams per kilogram of body weight) every day for eight weeks. In the animals that were scheduled to exercise, the supplementation was fed before the exercise.
3.5. Blood Sampling, Tissue Sampling, and Analysis
At the end of the eighth week and 48 hours after the last training session, the animals were deprived of food overnight (i.e., 12 hours of fasting) and then anesthetized by the intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). The heart tissue was removed by appropriate surgical tools (scissors and forceps). For biochemical measurements, a part of the tissue was immediately immersed in liquid nitrogen (- 196°C) for 10 minutes. The frozen tissues were then removed and kept at - 80°C until further analysis.
3.6. Molecular Analysis
The cardiac tissues of the animals, Rattus norvegicus, were evaluated by quantitative RT-PCR to evaluate the expression of the PPARG coactivator1 alpha (Ppargc-1α or Pgc-1α) and sirtuin 1 (Sirt1) genes. Actin alpha 2 (α-act) was applied as the reference gene. For this purpose, total RNA was extracted from the tissues by Qiagen RNeasy™ Mini Kit according to the manufacturer’s instructions. Then, the isolated RNA was quantified and qualified using the Thermo Scientific NanoDrop 2000 UV-Vis Spectrophotometer instrument. A certain amount of normalized RNA (≈ 200 ng) was used to synthesize cDNA by Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific) according to the manufacturer’s manual, and RT-PCR was carried out in Applied Biosystems StepOnePlus Real-Time PCR System. The PCR reaction, containing primers and template cDNA, was prepared by Applied Biosystems® SYBR® Green PCR Master Mix. The thermal cycle program was set up as an initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C, 57°C, and 72°C for 10, 15, and 30 seconds, respectively. Primers were designed for intron-spanning targets, as listed in Table 2.
| Genes and Primer Sequences | Tm (°C) | Product Size (bp) |
|---|---|---|
| Ppargc1a | 130 | |
| F: AAGAGCGCCGTGTGATTTAC | 58 | |
| R: TAGCTGTCTCCATCATCCCG | 58 | |
| Sirt1 | 111 | |
| F: AGTGATGACGATGACAGAGCA | 59 | |
| R: AGGATCGGTGCCAATCATGA | 59 | |
| α-Actin | 101 | |
| F: CATCATGCGTCTGGACTTGG | 59 | |
| R: TCTCACGCTCAGCAGTAGTC | 59 |
Abbreviations: Tm, melting temperature of primers in Celsius (°C); bp, base pair, the size of PCR products based on NCBI primer blast results; F and R represent forward and reverse primers, respectively.
3.7. Statistical Analyses
After obtaining raw data, the Shapiro-Wilk test was applied to check the normality of data distribution. The equality of variances was investigated by Levene’s test. After confirming the normality of the data and meeting the assumption of the equality of variances, mean and standard deviation were applied to describe the data. Also, one-way analysis of variance (ANOVA) and Tukey’s post-hoc test were used for the statistical analysis of biochemical findings and inter-group comparisons. Statistical tests were conducted in SPSS software, and P < 0.05 was considered statistically significant.
4. Results
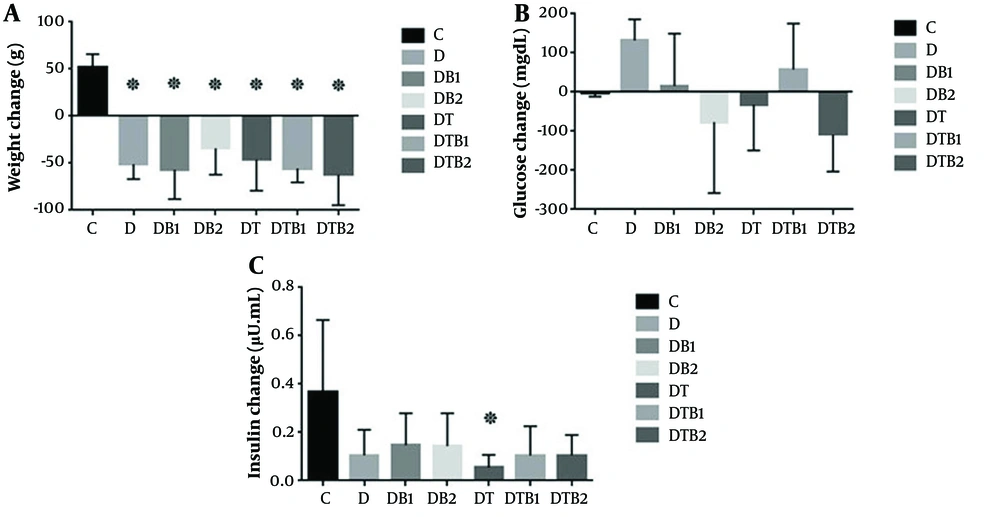
The results of one-way ANOVA revealed significant differences in weight changes between the study groups (P = 0.001). Tukey’s post-hoc test showed a significant weight loss in all groups compared to the control group (P = 0.001 for all). There were also significant differences between the groups in terms of insulin levels (P = 0.030). Tukey’s post-hoc test showed a significant reduction in insulin levels in the DT group in comparison with the control group (P = 0.017). No considerable difference was detected between the groups in terms of glucose levels (P = 0.080) (Table 3 and Figure 1).
| Variables and Group | Mean ± Standard Deviation | F | P-Value Intergroup |
|---|---|---|---|
| Weight (g) | 14.842 | 0.001 | |
| C | 343.49 ± 23.43 | ||
| D | 227.22 ± 32.03 a | ||
| DB1 | 213.74 ± 26.40 a | ||
| DB2 | 244.42 28.25 a | ||
| DT | 218.98 ± 29.58 a | ||
| DTB1 | 212.83 ± 19.06 a | ||
| DTB2 | 220.66 ± 22.85 a | ||
| Glucose (mg/dL) | 2.088 | 0.080 | |
| C | 99.01 ± 5.21 | ||
| D | 625.50 ± 23.18 | ||
| DB1 | 520.00 ± 101.58 | ||
| DB2 | 465.83 ± 77.61 | ||
| DT | 524.33 ± 60.00 | ||
| DTB1 | 549.33 ± 32.01 | ||
| DTB2 | 523.00 ± 73.10 | ||
| Insulin (µIU.mL) | 2.690 | 0.030 | |
| C | 0.367 ± 0.299 | ||
| D | 0.105 ± 0.106 | ||
| DB1 | 0.147 ± 0.133 | ||
| DB2 | 0.144 ± 0.135 | ||
| DT | 0.054 ± 0.053 a | ||
| DTB1 | 0.104 ± 0.121 | ||
| DTB2 | 0.106 ± 0.084 |
Abbreviations: C, healthy control; D, diabetic control; DB1, diabetic + 15 mg/kg berberine; DB2, diabetic+30 mg/kg berberine; DT, diabetic + exercise; DTB1, diabetic+exercise + 15 mg/kg berberine; DTB2, diabetic+exercise + 30 mg/kg berberine
a Indicates a significant decrease compared to the healthy control group.
Changes in weight, glucose, and insulin levels in the study groups (C, healthy control; D, diabetic control; DB1, diabetic + 15 mg/kg berberine; DB2, diabetic + 30 mg/kg berberine; DT, diabetic + exercise; DTB1, diabetic+exercise+15 mg/kg berberine; DTB2, diabetic + exercise + 30 mg/kg berberine; * indicates a significant decrease compared to the healthy control group)
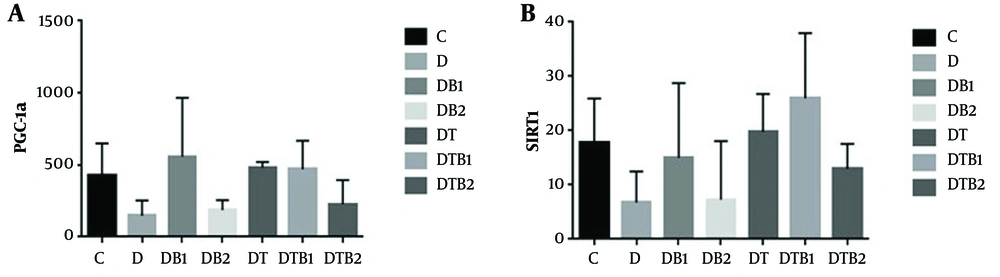
According to one-way ANOVA, no significant difference was detected between the groups in terms of the expression of PGC1-a (P = 0.071) or SIRT1 (P = 0.135) (Figure 2).
Changes in cardiac muscle expression of PGC-1α and SIRT1 in the study groups (C, healthy control; D, diabetic control; DB1, diabetic+15 mg/kg berberine; DB2, diabetic +30 mg/kg berberine; DT, diabetic + exercise; DTB1, diabetic + exercise + 15 mg/kg berberine; DTB2, diabetic + exercise + 30 mg/kg berberine; * indicates a significant decrease compared to the healthy control group)
5. Discussion
This study investigated the effects of aerobic exercise combined with the consumption of 15 and 30 mg/kg of berberine chloride on the expression of PGC-1α and SIRT1 in the cardiac muscle in diabetic rats. The findings of the study showed that eight weeks of aerobic exercise combined with berberine chloride supplementation at the doses of 15 and 30 mg/kg had no significant effect on the expression of SIRT1 and PGC-1α in diabetic rats’ heart tissues. This is the first study to investigate the interactive effect of exercise and berberine chloride supplementation on the expression of the PGC-1α and SIRT1 genes. However, there have been several studies on the combined effects of exercise and berberine chloride supplementation on other cardiac metabolic parameters. In one of these studies, Farhadfar et al. reported that four weeks of aerobic exercise combined with berberine chloride supplementation (50 mg/kg) significantly improved the levels of antioxidant enzymes in the cardiac tissue of diabetic rats (30). In another study, Sedighi et al. reported that six weeks of aerobic exercise combined with the use of berberine chloride (30 mg/kg) had a positive effect on oxidative stress markers in the heart tissue of rats with streptozotocin-induced diabetes (31). Ramezani et al. also confirmed the modulatory effects of exercise combined with berberine consumption on plasma levels of glucose, IL-6, and TNF-a in rats with type 1 diabetes (32). Thus, our findings appear to be inconsistent with the results of these three studies. This inconsistency could be due to the use of higher doses of berberine chloride in those studies. Furthermore, some studies have shown that the positive impact of exercise on antioxidant enzymes’ activity and lipid peroxidation can prevent the complications of diabetes and diabetes-induced oxidative tissue damage (33). However, our study on mitochondrial biogenesis indices showed that aerobic exercise at the intensity and duration executed in combination with berberine chloride consumption at the doses tested could not significantly change mitochondrial biogenesis.
In several studies, the effects of exercise alone on mitochondrial biogenesis have been investigated. In one of these studies, Shabani et al. observed a noticeable boost in the expression of PGC1α and VEGF in the cardiac muscle of healthy male rats following eight weeks of high-intensity intermittent training (34). In another study, Fathi investigated the prolonged effects of endurance training on PGC1α expression in the soleus muscle of male rats, and their results showed a significant increment in PGC1α expression in the experimental group compared to the control group (35). On the contrary, Ghiasi et al. reported that swimming exercise at an intensity of 46% of VO2max for eight weeks, while improving the metabolic condition of the subjects, had no significant impact on SIRT1 serum levels (36). This is while exercise can reduce the size of adipocytes and fat content in the fat tissue and enhance the levels of the enzymes involved in fat oxidation, as well as anti-inflammatory cytokines, accompanied by a decrease in the levels of pro-inflammatory cytokines. This hypothesis is supported by the reports showing reduced mitochondrial content in skeletal muscle and adipose tissue in several insulin-resistant models (37).
Physical activity promotes PGC1α production in the cardiac muscle tissue through factors such as nitric oxide (NO), AMP-activated protein kinase (AMPK) P38, and calcium/calmodulin-dependent kinase (CaMK). Subsequently, PGC1α augments the expression of nuclear respiratory factors (NRFs) and estrogen receptor alpha (α-ER), thereby increasing the expression of mitochondrial enzymes like cyclooxygenase (COX) and activating carbohydrate and fat oxidation enzymes (38). In vitro studies have also represented that PGC1α upregulation triggers the expression of oxidative isoforms and suppresses the expression of myosin heavy chain (MHC) isoforms, ultimately reducing myofiber transition. In a study, increased PGC1α expression in the skeletal muscle was noticed to occur two hours after exercise and remain at its peak for up to six hours (39). In another study, continuous endurance training was reported to stabilize PGC1α expression after 52 days (40). Swimming training has also been shown to increase the expression of PGC-1α and decrease the expression of HIF-1 in the cardiac muscle of rats under hypoxic conditions (41).
Despite causing sirtuins to elevate, sirtuin-induced signaling seems to have no role in the positive effects of regular exercise on the metabolic balance. As a regulatory protein, SIRT1 controls the metabolism of lipids and sugars, which is why it is widely expressed in different tissues (42). Also, SIRT1 plays several other roles, including deacetylating PPAR, FOXO, and PGC1α, each of which promotes multiple functions in different tissues (e.g., increasing gluconeogenesis, releasing glucose from the liver, inducing insulin secretion from pancreatic beta cells, and enhancing mitochondrial activity, fatty acid oxidation, and insulin activity in muscles) (43). Meanwhile, sirtuins are sensitive to nutrient depletion and are released in low-energy conditions to adjust metabolic pathways. Indeed, it has been suggested that therapeutic interventions that can activate SIRT1 (such as SIRT1 activators and exercise) should be investigated as potential treatments for obesity and metabolic diseases (44). Thus, the positive effects of exercise on insulin resistance and mitochondrial biogenesis can be mediated through 1- increasing the expression of glucose transporter type 4 (GLUT4) in the cell membrane by activating the intracellular message transmission pathway following contractions, 2- increasing the activity of insulin receptors, glycogen synthase, and protein kinase B, and 3- upregulating the factors involved in the insulin signaling cascade (45). Likewise, decreased SIRT1 expression and AMPK activity have been observed in the cardiac tissue following insulin resistance and inflammation (46). Therefore, the lack of change in SIRT1 in response to the exercise protocol in our study can be attributed to this reason. In a study by Sin et al. on adaptation to aerobic or endurance training, there was no significant change in the level of SIRT-1 protein in the cardiac tissue after 14 weeks, suggesting that SIRT-1 could lead to the activation of PGC1α through diacylation (47).
Several studies have also investigated the effects of berberine chloride supplementation alone on the metabolic balance of the cardiac tissue. Our study found no significant changes in the cardiac muscle’s expression of PGC1-a and SIRT1 after treatment with different doses (15 and 30 mg/kg) of berberine chloride hydrate. Previous studies, however, have noted that higher doses of berberine (100 mg/kg) can modulate mitochondrial biogenesis markers, as well as learning and memory functions in diabetic rats (25). After examining the effects of three doses of berberine chloride (25, 50, and 100 mg/kg), Chandirasegaran et al. stated that the dose of 50 mg/kg had the most prominent effects on blood glucose, plasma insulin, glycosylated hemoglobin, and body weight in diabetic rats (22). So, the lack of change in PGC1-a and SIRT1 after berberine chloride supplementation (15 and 30 mg/kg) in our study appears to be due to the low dosage. Berberine chloride has moderating effects on diabetes mellitus and insulin resistance through several molecular mechanisms, including modulatory effects on fat and glucose metabolism and antioxidant and anti-inflammatory activities (25). It has been shown that berberine consumption can reduce blood glucose and increase insulin sensitivity in rats with type-2 diabetes. The berberine-induced increase in glucose metabolism may be because of the stimulation of glycolysis and inhibition of mitochondrial oxidation. The findings of several studies have shown that berberine is a potent oral hypoglycemic agent with a positive impact on lipid metabolism (30-32). Berberine may also act as an alpha-glucosidase inhibitor, evidenced by reduced glucose transport across the intestinal epithelium (25). Moreover, all these effects can be attributed to the anti-inflammatory properties of berberine chloride as well (30).
5.1. Conclusions
To the best of our knowledge, this was the first study to investigate the effects of exercise combined with berberine chloride supplementation on mitochondrial biogenesis indicators in the cardiac tissue of diabetic animals. This study provides new insights into the synergistic effects of exercise and different doses of berberine chloride on the heart tissue of diabetic rats. Overall, our findings showed that aerobic exercise combined with berberine chloride hydrate supplementation at the two doses of 15 and 30 mg/kg did not exert a noticeable change in mitochondrial biogenesis markers in the heart tissue of diabetic rats. Therefore, this combination cannot be recommended as an effective intervention for improving cardiac mitochondrial biogenesis. However, since higher doses of berberine chloride and other types of exercise may yield different outcomes, further research needs to be conducted on this subject.