1. Background
Exercise causes the development of Reactive Oxygen and Nitrogen Species (RONS) by increasing oxygen consumption mainly through the electron transfer chain in mitochondria (1). Excessive production of RONS may induce oxidative damage, leading to oxidative stress damage in blood cells and plasma components (2). Malondialdehyde (MDA) is the original form of aldehyde produced through tissue lipid peroxidation and is widely known as a biomarker of oxidative stress and some serious clinical metabolic disorders (3). On the other hand, exercise reinforces anti-oxidative enzymes such as extracellular superoxide dismutase and catalase, which protect related enzymes against lipoprotein with high density (HDL) that hydrolyzes lipid peroxides against lipoproteins with low density (LDL) (4). Catalase is considered one of the most important antioxidant enzymes as it decomposes hydrogen peroxide into harmless products like water and oxygen. It is used as a therapeutic agent to overcome diseases related to oxidative stress (5). Total Antioxidant Capacity (TAC) expresses the antioxidant capacity of all antioxidants in a biological species and not just the antioxidant capacity of a unique combination (6).
Recent studies have shown that changes in environmental temperature may affect antioxidant status during exercise (7). During exercise, about 20 to 25% of released energy by muscular metabolism is utilized to do the work, while 75 to 80% is turned into heat, which should be released to make the body heat balance. This heat balance may be disrupted due to physical activity or staying in a hotter/colder environment. Increased body temperature may invoke the activation of leucocytes, cytokines, and markers of neutrophils during and after exercise, leading to systemic activation in a hot environment (8). On the effect of physical activity in the heat, previous studies claimed that hyperthermia augments oxidative stress induced by exercise and influences lipid oxidation markers selectively (9). Some evidence proves that increased central temperature resulting from separating the mitochondrial respiratory chain helps produce active oxygen species, increases iron cellular toxicity, produces Nitric Oxide, and augments xanthine oxidase (10). Combining exercise and heat imposes excessive stress on the body through many physiological and regulatory systems, like balancing oxidant and antioxidant factors. Findings on subjects with enough water supply show that increased central temperature is independently related to increased blood oxidative stress following exercise (11). Generally, the effects of doing exercise at high temperatures on the immune system (12), inflammation (13), and stress hormones (14) are well documented in the literature, while, to the best knowledge of the authors. There are few studies, particularly in Asian countries, on the effect of exercise at lower temperatures (14).
2. Methods
This semi-experimental study with a crossover design started after receiving the IR ID code.USB.REC.1400.088 from the ethics committee of Sistan and Baluchestan University, in which two pre-test and post-test groups were selected to study the effect of a week of prolonged running in hot and cold environments on some saliva antioxidant responses. To do so, 10 participants were selected via the purposive sampling method from eligible available people and were placed into one single group. Inclusion criteria were the total health condition of the participants- no history of cardiovascular disease, asthma, diabetes, and any bone fracture, the age range of 18 to 22 years, non-participation in regular exercise programs in the past 2 years, and no smoking. Exclusion criteria included a history of cardiovascular diseases, asthma, diabetes, and bone fracture and the unwillingness of the participant to continue with the procedure.
2.1. Participants
Participants included 10 young boys aged 16.0 (±0.32) years with 65 (±3.59) kg weights and 22.5 (±1.49) kg/m2 BMI who were screened according to the predefined inclusion criteria of the study procedure and were selected via convenience and purposeful sampling method.
2.2. Exercise Protocol
The exercise protocol in this study included a session of prolonged running (1.5 miles = 2400 m) with 50 to 60 % maximum heartbeat on the treadmill. The first session was in a warm environment (31°C), while the second one was in a cold environment (15°C). Five to ten minutes in each session was devoted to warming up at the beginning, and 5 to 10 minutes was devoted to cooling down. Exercise intensity was controlled using a pulse oximeter.
2.3. Saliva Sampling
Saliva was sampled following 8 to 10 hours of fasting, tooth brushing, and washing out with distilled water in a sitting position on a chair without any stimuli with the head inclined to the front. The saliva samples were centrifuged (in 7500 rounds/minute for 10 minutes) and were placed in -4°C condition until laboratory procedures started.
The total Antioxidant Capacity of Saliva was measured using the FRAF2 Method. This method is centered on the species' ability to revive ferric ions (Fe+3) to Fro ones in the presence of a substance called Tripyridyl-s-Thiazine; moreover, the revitalizing capacity of each species was measured by increasing the density of the above-mentioned complex by a spectrophotometer. Measuring Malondialdehyde levels in saliva using a method focusing on the reaction to Thiobarbituric Acid (TBA) was performed at 96°C to 100°C temperatures. In this method, MDA and TBA mutually affect each other, resulting in a pink complex. To do so, 100 µ of the sample was diluted with 900 µ of distilled water, adding 500 µ of TBA reagent. In order to produce 100 ml of reagent, 0.5 g of NaOH and 0.67 g of Thiobarbituric Acid were mixed with 100 ml of Acetic Acid. The sample, including the reagent, was heated for an hour at 90°C temperature. After cooling down, the samples were centrifuged for 10 m in 4000 rounds. Then the covering layer was separated, and the optical absorption of the samples was read using a spectrophotometer at a 535 nm wavelength. The results were collected following comparison with the standard table, and MDA levels were measured using µmol/ml. To measure catalase enzyme activity level (CAT), 50 mmol of buffer phosphate catalase (pH = 7) was combined with 10 mmol of hydrogen peroxide. Then two quartz cuvettes were selected, and 500 µL of buffer phosphate and hydrogen peroxidase were added to the blank cuvette, 250 µL of buffer phosphate and hydrogen peroxidase and 55 µL of saliva were added to the sample cuvette and were recorded kinetically at 240 wavelengths for 1 minute with 5 s intervals using a spectrophotometer. Finally, the results were divided by 39.4 to calculate catalase enzyme activity.
2.4. Statistical Analysis
To determine the normality of the data, Kolmogorov- Smirnov test was applied, while repetitive measures ANOVA was applied to compare changes in studied variables. The significance level is considered as P ≤ 0.05.
3. Results
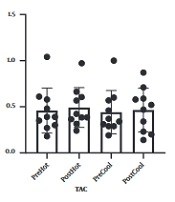
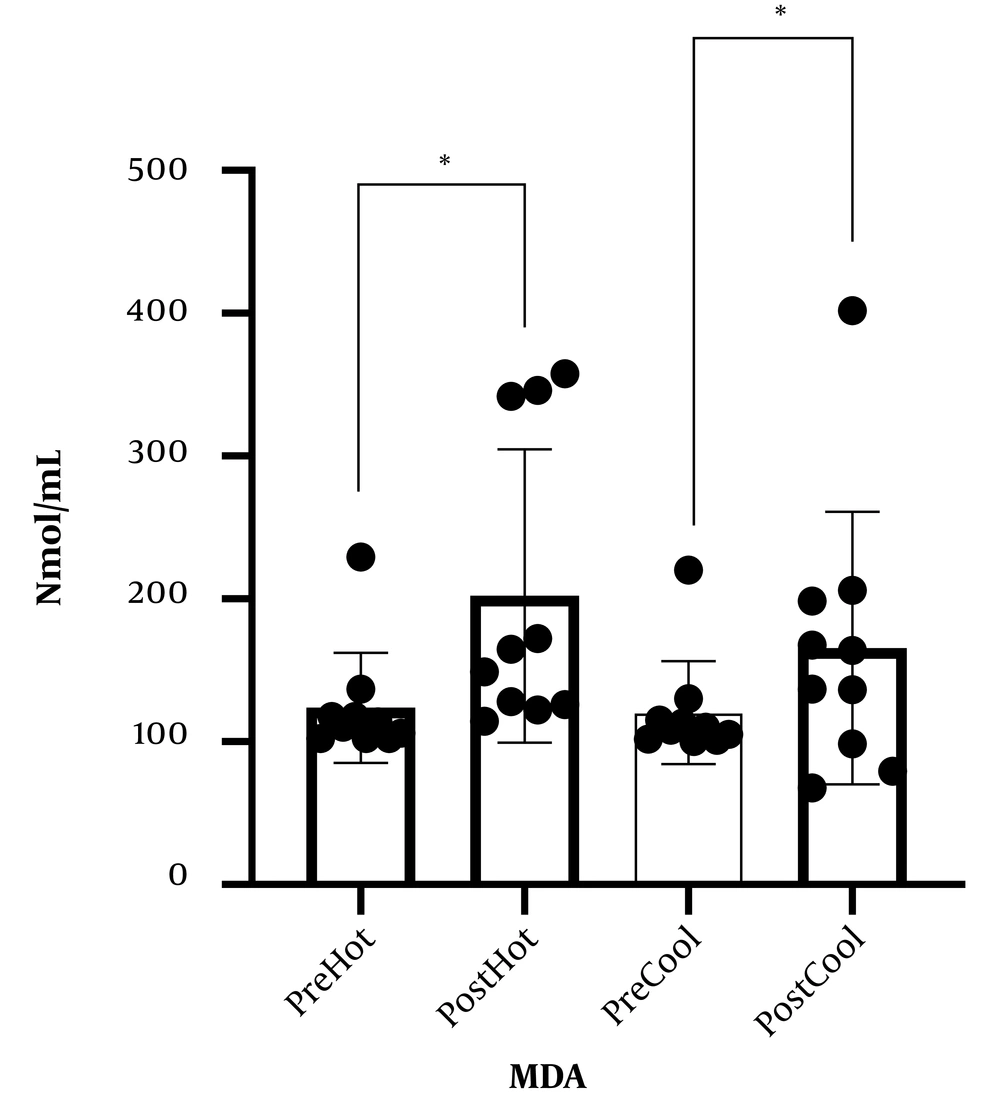
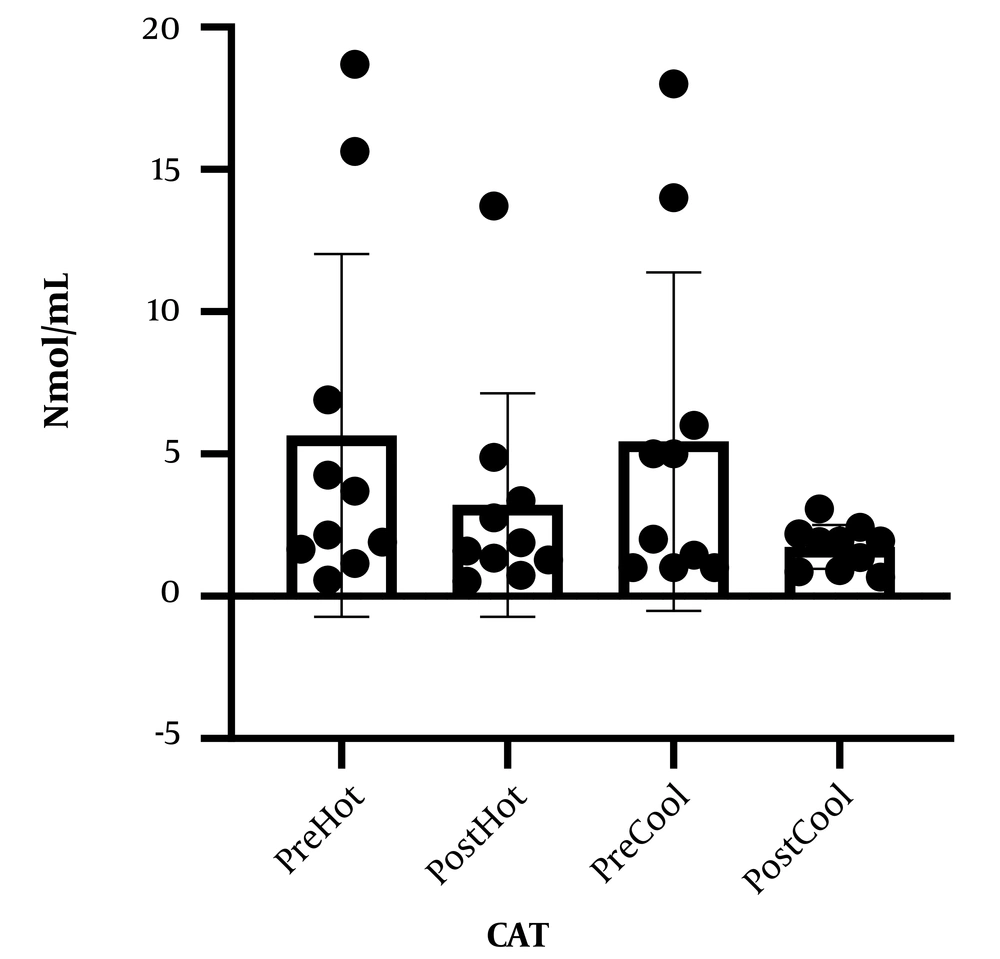
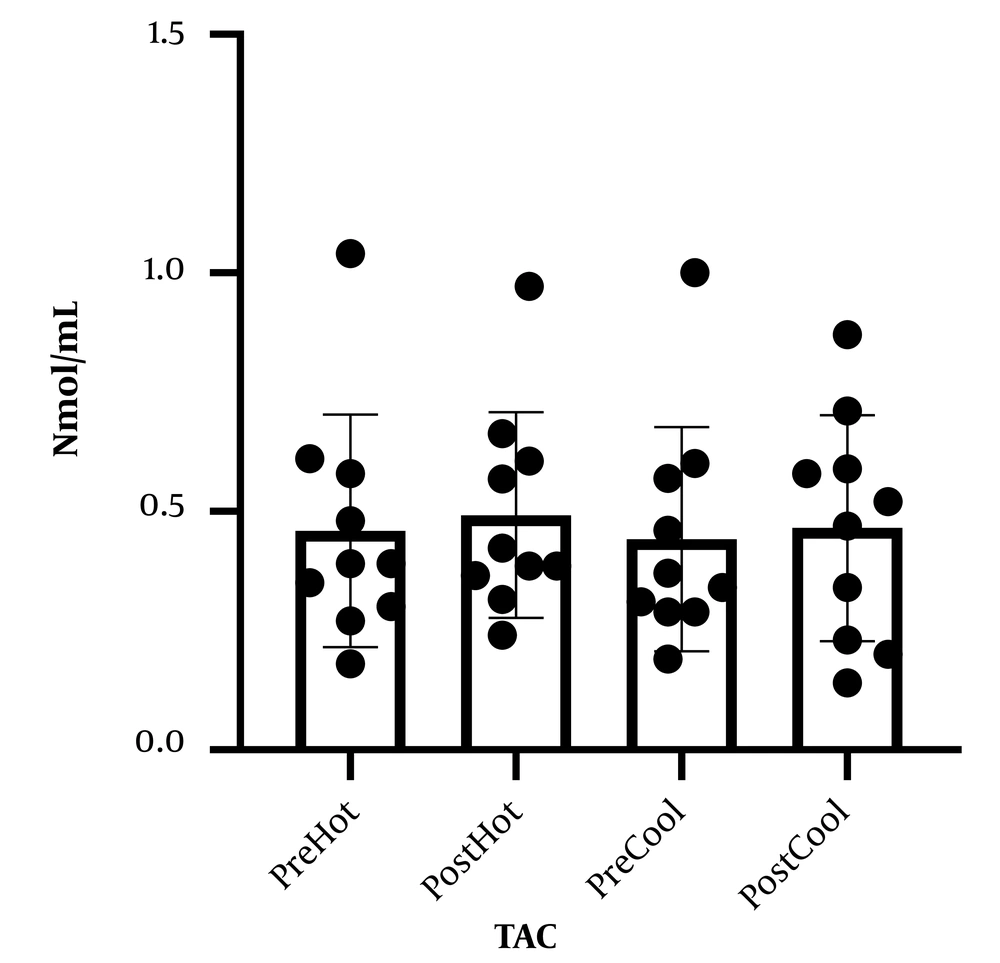
Table 1 represents the personal information of participants. Comparing malondialdehyde levels, catalase, and total antioxidant capacity, before and after involvement showed that malondialdehyde levels increased after prolonged running in cold and warm environments. Although catalase levels and total antioxidant capacity increased following a prolonged running session in cold and warm environments, these changes were not statistically significant. In addition, comparing studied variables following involvement in cold and hot environments, no significant difference was observed (Figures 1 - 3).
| Variables | Number | Mean ± Standard Deviation |
|---|---|---|
| Age, y | 10 | 16 ± 0.32 |
| Height, m | 10 | 1.70 ± 0.05 |
| Weight, kg | 10 | 65 ± 3.59 |
| BMI, kg/m2 | 10 | 22.5 ± 1.49 |
4. Discussion
What explains the novelty of this study is its focus on the effect of a session of prolonged exercise along with environmental pressure (heat and cold) on oxidative stress and antioxidants. We presume that oxidative stress induced by exercise increases in hot weather. In addition to proving this hypothesis, our findings showed that oxidative stress in non-athlete people rose following exercise in a cold environment in a way that there was no difference between this rise and its augmentation in a hot environment. Moreover, physical activity causes invigoration of the body's antioxidant potential; our findings also demonstrate that the antioxidant defense affected by prolonged running in cold and hot environments does not change. The present study's findings correspond to previous studies (13, 15, 16). In this regard, Sureda et al. and Cosio-Lima et al., by comparing inflammatory factor levels after exercise in a hot environment, reported an increase in inflammatory factor secretion following activity in a hot environment compared to a cold environment (13, 17). Our findings align with the findings of a study that showed that increased inflammatory factors might cause the rise of oxidative stress (18). To elucidate these results, it is shown that exercise and activity in a warm environment, compared to normal temperature, causes some disorders in the immune system through increasing blood circulation and secretion of stress hormones and catechols (14). Nevertheless, few studies support our findings on the effect of physical activity in a cold environment on oxidative stress in a way that McFarlin and Mitchell in line with our findings, expressed that doing exercise in hot (38°C) and cold (8°C) environments, involves stress response which may cause changes in the immune system (19). However, Quindry et al. reported that oxidative stress in a hot environment (33°C) increased while it did not increase following exercise in a cold (7°C) environment (16). To explain our results and what Quindry et al. found, cold environment temperature is considered 7°C while our study considered the cold temperature as 15°C, based on the previous findings of others it has been shown that lower environment temperature may induce oxidative stress, which will be influenced by an increasing body central temperature (15). Moreover, Mestre-Alfaro et al. studied Malondialdehyde changes following a session of running in a warm environment (30°C - 32°C) and a cold environment (10°C - 12°C) in resistance athletes. They declared no significant changes in malondialdehyde levels following exercise (8). In the study performed by Mestre-Alfaro et al., the participants were a group of trained resistance runners for whom the exercise intensity of a running session was not enough to cause oxidative stress, as they were participants with that level of physical readiness because it has been shown that short periods of exercise in hot and cold do not cause significant oxidative stress in trained individuals (20). Induced oxidative stress of exercise depends on participants' intensity, duration, environmental condition, and physical readiness (13, 21), while participants in our study were non-athlete individuals. Our findings have shown that antioxidant defense does not change in people who run in cold and hot environments. In accordance with our findings, Ibrahim et al. announced that the saliva lysosome response (salivary defense system indicator) to physical activity in hot and cold environments is the same (14). However, former studies have shown that cold weather can increase antioxidant defense in participants (22, 23). To clarify these results, the cold-water temperature can be pointed out. In a study, Lubkowska et al. (23) studied the effect of winter swimming on the antioxidant system in which the water temperature was about 4°C while it was considered between 10°C to 14°C in the performed study by Eimonte et al. (22). It seems lower environmental temperature with imposing higher stress causes a change in the immune system (antioxidant) (19).
These findings demonstrate that physical activities, whether in cold or warm environments, might cause ineffectiveness of body antioxidant potential in non-athlete persons and increase oxidative stress. Klarod et al. expressed that doing short-term intense exercise in hot or cold places does not induce oxidative stress in trained persons (athletes) (20).
4.1. Limitations
Measuring participants' body central temperature was not fulfilled because there was no possibility of receiving the necessary laboratory facilities. Nevertheless, presenting changes in the selected enzyme levels, oxidative stress, and antioxidant defense made us able to determine the effect of a week of physical activity in cold and warm environments on enzymes well. Also, we did not measure and report the humidity level in the laboratory, but both stages of the experiment were carried out under the same temperature and humidity conditions.
4.2. Conclusions
According to the results, it can be concluded that non-athlete persons should have continuous physical activity to fulfill the profitable consequences of aerobics. At the same time, individual exercise sessions without invigorating antioxidant defense increase the danger of oxidative stress in these people.