1. Background
Health promotion in today's societies helps people change their lifestyles to move towards a favorable health status. In recent decades, it has been shown that exercise and regular physical activity can have many beneficial effects and effectively improve health and treat many diseases (1-3). Skeletal muscles are heavily involved during prolonged exercise, and their main source of energy is fats (4). These fuels include triglycerides and plasma-free fatty acids (5). To maintain normal blood fat levels, the absorption of free fatty acids by skeletal muscles during exercise is very important and can prevent some diseases in healthy people (6). In addition to the oxidation of free fatty acids in muscles and from a skeletal pathological point of view, it can be contended that the oxidation of free fatty acids is important for athletes as an important source of energy (7). Many regulatory processes are involved in the oxidation of fatty acids in skeletal muscles, some related to the transfer of free plasma fatty acids into muscle cells, carried out by transporters in cell plasma membranes. On the other hand, on the surface of the mitochondrial membrane, there are fatty acid transporters connected to CoA, which play a role in the entry of free fatty acids into the mitochondria (8). Among fatty acids' plasma membrane transporter proteins, the FAT/CD36 transporter, also called CD36, is located on the plasma membrane of some tissues such as skeletal muscles, heart, liver, adipose, and the small intestine (9). In the heart and skeletal muscles, there are intracellular sources of this protein that can be translocated to the plasma membrane to regulate the uptake of long-chain fatty acids. The activation of this protein occurs through several signaling cascades, including AMPK, CAMK, ERK1/2, and insulin (9). To prove the effective role of CD36 in fatty acid oxidation, Coburn et al. showed that the rats with CD36 deficiency had a 60% reduction in the transfer of fatty acids into cells such as skeletal muscles, heart, and fat cells, and their ability to perform endurance sports activities was reduced. Moreover, researchers have demonstrated that muscle contraction activity increases the transfer of CD36 from the cytoplasm to the plasma membrane (10). It has also been demonstrated that CD36 is also found on the mitochondrial membrane. Although its location and functional role in this organelle has not yet been fully determined, there is a hypothesis that CD36 with the CPT protein can affect the transport of long-branched free fatty acids to mitochondria (11). The CPT or carnitine palmitoyl transferase complex includes CPT1 and CPT2, which play an important regulatory role in the transfer of fatty acids. CPT1 is located on the outer surface of the outer membrane of the mitochondria and carries out the transfer of various chain fatty acids in the form of acyl-CoA. The produced acyl-CoA carnitine can penetrate the inner membrane of the mitochondria, where the acylcarnitine/carnitine translocase protein carries out its transfer. Subsequently, acylcarnitine enters the mitochondrial matrix and participates in the β-oxidation cycle by the CPT2 protein on the inner mitochondrial membrane (12). Recently, two isoforms of CPT1 have been identified in different tissues, namely L-CPT1 and M-CPT1. As the primary isoform, L-CPT1 is mainly expressed in the liver, kidney, lungs, pancreas, and brain, while M-CPT1 isoform is mainly found in skeletal muscles and adipose tissues (13). The oxidation of free fatty acids continues inside the mitochondria in a cyclic process called the β-oxidation cycle, which takes place with 4 different enzymes, and 3-hydroxyacyl CoA dehydrogenase (HADH) has been identified as the most important enzyme of this cycle. HADH catalyzes the third stage of β-oxidation reactions by reducing NAD to NADH and producing 3-ketoacyl CoA, which is expressed in large amounts in the heart, liver, adipose tissue, and pancreas (14). The rate of oxidation of fatty acids during exercise is affected by various factors in metabolic pathways. Some of these factors are related to systemic factors (including the maximum oxygen consumption of the body, the oxidative power of muscles, types of muscle fibers, and the size of energy reserves), and others are related to special conditions during exercise. Factors related to specific conditions include intensity, duration, and type of exercise, sympathetic nervous system activity, and substrate availability (7). The type, duration, and intensity of exercise training can affect fatty acid transporters differently (15). Most of the previous research has been done on endurance training. Novel training methods, such as resistance and interval training, are becoming increasingly popular (16). Talanian et al. showed that 6 weeks of intense interval training on healthy women increased the content of fatty acid transporters in skeletal muscles (17). Hoshino et al. also examined the effect of intense interval training on the content of fatty acid transporters. Intense interval training increased CD36 levels in slow and fast twitch fibers (18).
Recently, some supplements have been demonstrated to increase fat metabolism and cause long-term adaptations in promoting fat metabolism (19-21). Grape seed oil has many therapeutic uses due to its health benefits. It has a high content of unsaturated fatty acids, phenolic compounds, phytosterols, and vitamins. Grape seed oil contains high amounts of linoleic acid, an essential fatty acid. The effects of grape seed oil on improving lipid and energy metabolism disorders and insulin resistance are well-documented. It is also demonstrated that grape seed oil can improve insulin sensitivity and reduce the production and concentrations of low-density lipoprotein (LDL) particles in men with dyslipidemia, which may be due to its high polyphenol content (22). Despite several studies on the effects of nutritional supplements and exercise (23-26), no study has yet investigated the simultaneous effect of resistance and high-intense interval training along with grape seed oil on fatty acid transfer indicators.
2. Objectives
The purpose of this research was to investigate the effect of two types of resistance and high-intensity interval training along with the supplement of grape seed oil on the expression of CD36, CTP-1, and HADHA genes involved in the oxidation of free fatty acids in the gastrocnemius and soleus muscles of male rats.
3. Methods
3.1. Animals and Design Study
The research committee of Mazandaran University approved this research (IR.UMZ.REC.1401.061). Thirty male Wistar rats (250 ± 25g) were obtained from the Pasteur Amol Institute research center and transferred to the animal laboratory of the Faculty of Sports Sciences. Animals were kept in standard polycarbonate cages in an environment with an average temperature of 22 + 1.4°C, humidity of 55% + 4, and a light-dark cycle of 12:12 hours, and they were freely exposed to water and chow. After a week of adaptation to the laboratory environment, they were divided into 6 groups as follows: the control group, the grape seed oil group, the resistance training group, the high-intensity interval training (HIIT) group, the HIIT-grape seed oil group, and the resistance- grape seed oil group.
3.2. Grape Oil Extraction and Its Application
To extract oil from grape seeds, we first washed the purchased white grapes and then separated the seeds from other parts of the pulp. For this purpose, we passed the pulp through a rotating cylinder until only the kernels remained. Then, to dry the kernels, we put them in a dryer at a temperature of 40°C for one day, and then the oil was extracted by a press machine using the cold-press method. To separate the non-oily parts, we put the extracted oil through the sedimentation stage, and grape seed oil was fed by gavage to the animals one hour before training at a dose of 3.7 g/kg of body weight of the rat.
3.3. Training Protocols
3.3.1. High-intensity Interval Training
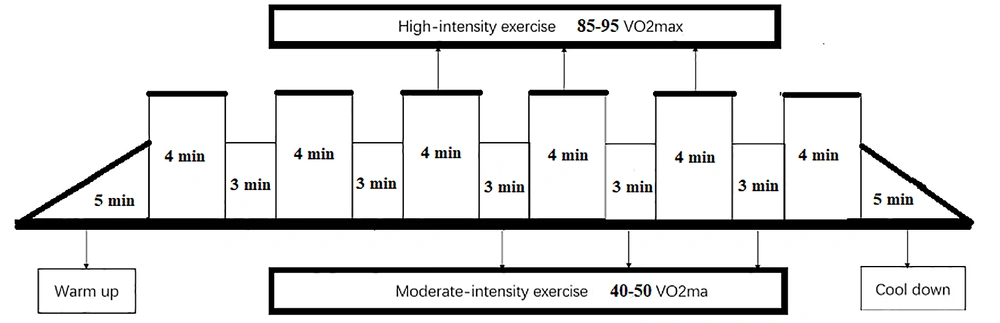
In the familiarization phase, the animals learned how to run on a treadmill for 10 minutes at a speed of 10 meters per minute 3 days a week for two weeks. Then, in the main training phase, after 5 minutes of warming up at 40% of the maximum oxygen consumption, the rats performed 39 minutes of interval activity in a form that included six stages, each lasting 4 minutes with an intensity of 85 - 95% of the maximum oxygen consumption and five 3-minute active rest period with an intensity of 40 - 50% of the maximum oxygen consumption between the activity periods. At the end of the exercise, the rats performed the cooling phase on the treadmill for 5 minutes. The running capacity test was performed before starting the exercises for each animal to evaluate the maximum running capacity. In this manner, the mice of the test group started running on the treadmill at a speed of 6 m/min and for 5 minutes with a 25% incline; then, after every two minutes, the speed of the treadmill was increased by 0.5 m/min until the mice reached the point of failure. Failure was defined as the point where the animal remained on the shocker part of the treadmill for more than 5 seconds despite being encouraged to run. The maximum running capacity was considered a stage where the animal could run for at least 50 seconds. To make the conditions the same for the experimental and control groups, the control group ran for 10 minutes a day and once a week on a treadmill at 10 meters per minute. During this period, the environmental conditions for the control group were completely similar to the experimental group (Figure 1) (27).
3.3.2. Resistance Training
In the resistance training phase, a 1-meter-high ladder was used, which had 26 steps and was placed at an angle of 80 degrees. The rats lifted the training weights attached to their tails with glue and clamps without any shock. The whole training program consisted of 2 stages: In the phase of adaptation to the laboratory environment, the rats were trained for 2 weeks, three days a week (4 - 5 repetitions each session with a 2-minute rest between repetitions), though the above method they learned to climb the ladder without carrying weights, and no electric shock or other stimulation was used. The second stage lasted for 8 weeks, 3 sessions a week. The beginning of each session consisted of four steps of climbing the ladder. It included increasing loads of 50, 75, 90, and 100% of the maximum weight-carrying capacity with a two-minute rest between activities. After the rats could carry 100% of the maximum weight-carrying capacity, 30 grams of weights were added to the previous load, and the same process continued until the rats reached exhaustion. Maximum weight-carrying capacity was measured after weekly weighing of mice. In this manner, 75% of the body weight of each mouse was determined, 30 grams were added to it, and the mice lifted this load on the ladder; the increase of 30 grams of load continued until the mice could climb. The failure point was determined as the maximum weight-carrying capacity. To perform the warm-up and cool-down steps, climbing the ladder without weights was considered twice before and after each training session (28).
3.4. Sampling
Seventy-two hours after the last training session, to eliminate the acute effect of training and after 12 hours of fasting, rats were anesthetized by intraperitoneal injection with a combination of ketamine (50 mg/kg) and xylazine (5 mg/kg). The rats' gastrocnemius and soleus muscles were removed from the right sides of the tissue. After washing, they were placed in an autoclaved microtube. Then, all tissues were immediately frozen using liquid nitrogen and transferred to a -80°C refrigerator.
3.5. Molecular Measurements
For molecular measurements, RNA extraction was first performed from the gastrocnemius and soleus muscles by an mRNA-isolating kit according to the manufacturer's protocol (Denazist, Mashhad-Iran). The quantity and quality of the extracted RNA solution were assessed using a NanoDrop device. cDNA synthesis steps were performed by a cDNA synthesis kit (Yekta Tajhiz, Iran) according to the manufacturer's protocol. The synthesized cDNA was then used to perform the reverse transcription reaction. The designed primers related to HADHA, CPT1, and CD36 genes are presented in Table 1. The 2-ΔΔCT calculated the expression level of the target genes.
| Gene | Sequence (5’-3’) | Accession | Product |
|---|---|---|---|
| HADHA | |||
| F | GTATGGTGCTCAGAAGGTAGTGG | NM_130826.2 | 97 |
| R | GTTAGCAAGGTCGCGGAGTAG | ||
| CD36 | |||
| F | AGTGGCAAAGAATAGCAGCAAGA | NM_001109218.1 | 157 |
| R | AGACAGTGAAGGCTCAAAGATGG | ||
| Cpt1 | |||
| F | ACACCAAAACATGTACCGCCTA | NM_013200.2 | 107 |
| R | CCAGGAAAGGAGACCTAACCC | ||
| β-actin | |||
| F | GTGTGACGTTGACATCCGTAAAGAC | NM_031144.3 | 119 |
| R | TGCTAGGAGCCAGGGCAGTAAT |
3.6. Statistical Analysis
All statistical analyses were done by SPSS version 16 software. Data were shown as mean ± standard error of the mean and analyzed by descriptive and inferential statistics. The normal distribution of the data was investigated using the Kolmogorov-Smirnov test, and one-way variance analysis and LSD follow-up test were used to determine the difference between the groups. The significance level was considered at P < 0.05.
4. Results
4.1. Body Weight
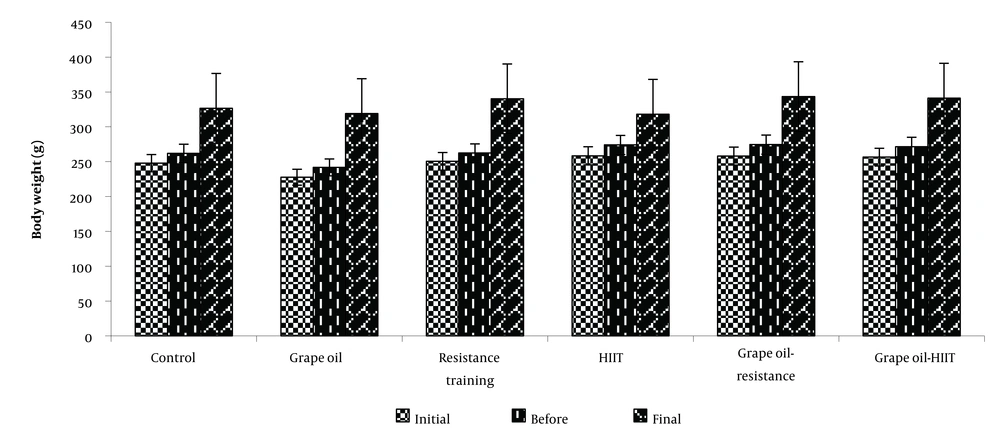
Table 2 shows the mean ± SEM of body weight in the different groups in the three initial stages, before and after starting the exercises. The results of the analysis showed that there was no significant difference between the groups at the end of each period (Figure 2).
| Control | Grape Oil | Resistance Training | HIIT | Grape Oil- Resistance | Grape Oil - HIIT | |
|---|---|---|---|---|---|---|
| Initial weight (g) | 247.87 ± 25.38 | 227.62 ± 31.50 | 250.75 ± 24.52 | 258.37 ± 24.59 | 258.12 ± 20.23 | 256.5 ± 21.77 |
| Before the protocol (g) | 261.87 ± 21.25 | 241.75 ± 33.42 | 262.37 ± 25.85 | 274 ± 23.18 | 274.5 ± 13.63 | 271.5 ± 22.09 |
| Final weight (g) | 326.5 ± 24.19 | 319.12 ± 25.45 | 340.62 ± 20.37 | 318.37 ± 21.11 | 343.37 ± 19.46 | 341.16 ± 39.19 |
4.2. The Expression of CD36 in the Soleus and Gastrocnemius Muscles
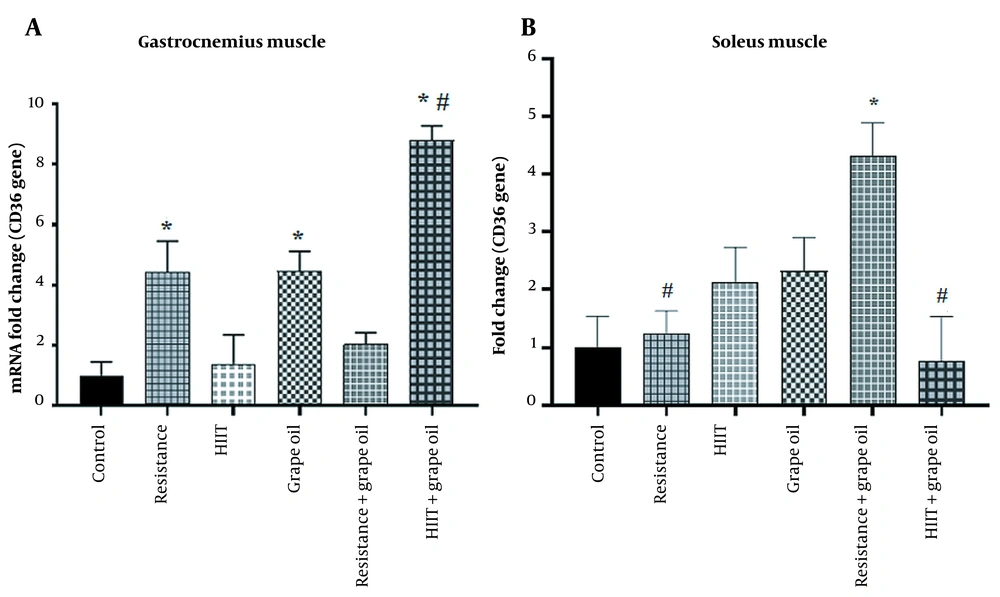
The results of CD36, CPT1, and HADHA expression in the soleus and gastrocnemius muscles are presented in Figures 3, 4, and 5. The expression of CD36 in the gastrocnemius muscle significantly increased in the resistance group (P = 0.043), the grape seed oil group (P = 0.042), and the grape seed oil and intense interval exercise (P = 0.005) group compared to the control group. Moreover, this expression in this muscle significantly increased in the grape oil-HIIT group compared to the HITT group (P = 0.014). The CD36 expression in the soleus muscle significantly increased in the resistance-grape oil group compared to the control group (P = 0.021), the resistance group (P = 0.047), and the HIIT-grape oil group (P = 0.016) (Figure 3).
The mean ± SEM of the expression of CD36 in the gastrocnemius (A) and soleus (B) muscles. * Shows significant difference with the control group. # Panel A shows a significant difference with the HIIT group (P < 0.05). # in the panel B shows significant difference with the resistance + grape oil group (P < 0.05).
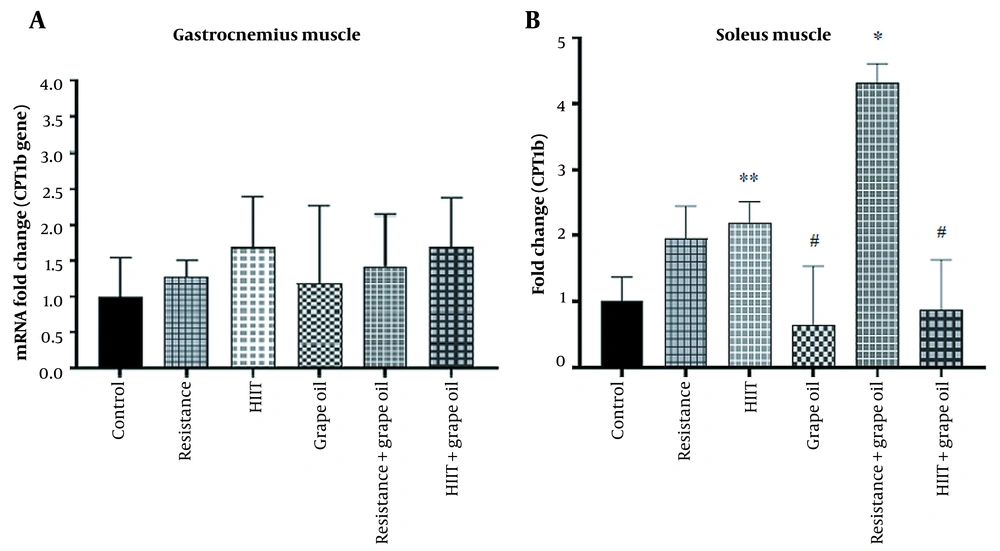
4.3. The Expression of CPT1 in the Soleus and Gastrocnemius Muscles
Although the expression of the CPT1 gene in the gastrocnemius muscle increased in all groups, they had no significant changes. The expression of the CPT1 gene significantly increased in the resistance-grape oil group compared to the control (P = 0.027), the grape oil (P = 0.005), and the HIIT-grape oil (P = 0.029) groups. Moreover, its expression significantly increased in the HIIT group compared to the grape oil group (P = 0.03) (Figure 4).
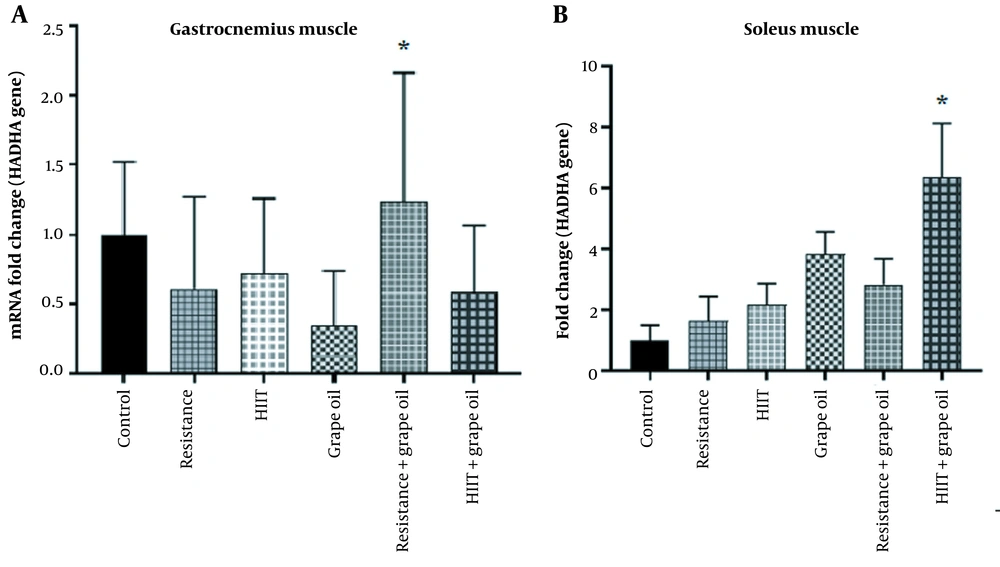
4.4. The Expression of HADHA in the Soleus and Gastrocnemius Muscles
The results showed that although the expression of HADHA in the gastrocnemius and soleus muscles significantly increased in the resistance-grape oil and the HIIT- grape oil groups, respectively (P = 0.05), its expression in the other groups had no significant changes (Figure 5).
5. Discussion
The results demonstrated that exercise and consuming grape seed oil can assist in weight management in the studied animals. It seems that a combination of resistance training and HIIT, along with the consumption of grape seed oil, could affect the weight of the subjects of this study by controlling other factors affecting food intake and energy metabolism. In this regard, the important factors involved in lipid oxidation, i.e., those that control the entry of fatty acids into cells and mitochondria, were investigated. Our results demonstrated that resistance training, grape seed oil, and HIIT, along with the consumption of grape seed oil, could increase the expression of the CD36 gene in the gastrocnemius muscle. In contrast, the expression of the CPT1 gene did not show any alternation. Moreover, resistance training and the consumption of grape seed oil could increase the expression of CD36 and CPT1 genes in the soleus muscle. Changes in the expression of the HADHA gene in the soleus and gastrocnemius muscles had different results depending on the type of exercise training, and the expression of the HADHA gene was increased in the soleus and gastrocnemius muscles by HIIT and grape seed oil, and resistance training respectively.
The transfer of free fatty acids into cells, especially muscle cells, occurs through CD36. After muscle contraction, CD36 immediately translocates into the plasma membrane (29). Schenk and Horowitz reported that regular endurance training increased levels of CD36 in muscle cells, and it could increase free fatty acid oxidation levels by 23% at rest. In addition, increased levels of CD36 remain high up to 3 days after exercise (30). Moreover, it was reported that the level of CD36 increased through endurance training. This cellular response can be an adaptive mechanism to provide energy via free fatty acid oxidation (30-32). However, it is important to adopt new training types, such as resistance and high-interval training, and investigate their effect on transporters of free fatty acids. Our results indicated that although doing HIIT could increase the expression of CD36 in the soleus muscle, this increase was not significant. However, its expression had no significant change in the gastrocnemius muscle. Moreover, resistance training could increase the expression of CD36 in the gastrocnemius muscle but not in the soleus muscle. In line with our results, Hoshino et al. examined the effect of 4 weeks of intense interval training (5 days a week, 10 bouts of 1-minute intense activity of running on a treadmill at a speed of 35 m/min with 2-minute rests) on the fast and slow twitch fibers of the gastrocnemius muscle. They found that total muscle CD36 levels increased in high-intense interval training, which was 27% and 22% for fast and slow twitch fibers, respectively (18). Talanian et al. showed that high-intensity interval training for 2 and 6 weeks increased the cytoplasm content of CD36 and mitochondrial content (17). Moreover, Ibrahimi et al. showed that the overexpression of CD36 could increase the rate of oxidation of free fatty acids and improve insulin sensitivity (33). Bonen et al. demonstrated that the expression of CD36 in the oxidative muscles is higher than in the glycolytic muscles (34). Barzegar et al. reported that 4 weeks of HIIT increased the expression of CD36 in the soleus muscle (35). However, in our study, no significant changes were observed in the CD36 expression in the soleus muscle by HIIT. This can be attributed to the intensity, type, and duration of exercise and the type of slow-twitch and fast-twitch skeletal muscle fibers. Moreover, Hoshino et al. (18), Gibala et al. (36), and Terada et al. (37), who investigated the effect of HIIT on fatty acid oxidation, all reported an increase in PGC-1α, mitochondrial content, and free fatty acid transporters. However, Egan et al. showed that HIIT can increase the transcription rate of PGC-1α and mitochondrial biogenesis by activating AMPK and CAMK and increasing the oxidation of free fatty acids (38). Others demonstrated that resistance training affects the oxidation of free fatty acids by activating the mTOR signaling pathway and phosphorylating 4E-BP1 and S6K1 proteins, resulting in protein synthesis and increasing muscle mass (39, 40). Therefore, measuring the level of PGC-1α in the present study and investigating its correlation with CD36 could be helpful in this regard. Moreover, the measurement of CD36 in the present study was at the gene expression level, and it was different from the measurement of some research conducted at the cytoplasmic and post-translational levels (41-43).
Comparing the results of CD36 gene expression in soleus and gastrocnemius muscles demonstrated that they increased significantly in the two groups of resistance training with grape seed oil and intermittent training with grape seed oil, respectively. In contrast, resistance and interval training alone could not have this effect. Therefore, the effect of grape seed oil supplements in these groups is clearly defined. Li et al. showed that grape seed oil reduced insulin and leptin resistance and improved energy metabolism in rats fed with a high-fat diet. In addition, grape seed oil could decrease the serum levels of fasting blood glucose, triglyceride, and cholesterol (22). Kim et al. showed that grape seed oil supplementation caused a significant reduction in total cholesterol (44). Hushang and Kim found that grape seed extract could increase fatigue time in basketball players due to polyphenolic compounds (45). These results clearly show that grape seed oil has a significant effect on the expression level of this gene and can change the expression of this gene concerning sports training. These results show that grape seed oil can provide the required energy through changes in the gene expression content of transporters during exercise. However, this effect on the number of their transporters or other factors requires more comprehensive research.
Our research demonstrated that the CPT1 gene in the gastrocnemius muscle had no significant change in all groups. The expression of the CPT1 gene in the soleus muscle significantly increased in the resistance-grape oil group and HIIT group. In line with our research, Carnevali et al., who investigated the effect of HIIT on the expression of the CPT-1 gene and its activity in the gastrocnemius muscle of male rats, reported that the expression of the CPT-1 gene had no significant change in the HIIT group. In contrast, its activity increased and enhanced mitochondrial lipid transport capacity (46). In contrast to the results of our study, Shahouzehi et al., who investigated the effect of HIIT along with L-carnitine supplementation on the expression of the CPT-1 gene in the liver of male rats, found that this expression increased in the HIIT along with L-carnitine consumption group (47). This contradiction may be attributed to the fact that the expression of the CPT-1 gene in different tissues can be affected by different mechanisms (48). Moreover, an increase in the expression of the PGC-1α gene was observed in the liver tissues of male rats subjected to intense interval exercise and L-carnitine supplementation. Therefore, it can be concluded that considering the role of PGC-1α in the transcription of factors involved in the metabolism of fats, there may be a possible relationship between PGC-1α and CPT-1 gene expression. It should be noted that the expression of the CPT-1 gene in oxidative and glycolytic muscles can be different due to the differences in the levels of oxidative enzymes (49) and the mitochondrial content (50), which requires further investigation. It is demonstrated that the activity of CPT-1 at the cytoplasmic level can be influenced by malonyl-CoA, Acetyl-CoA carboxylase, and AMPK. In resting muscle cells, CPT-1 activity is inhibited in the presence of malonyl-CoA, while during exercise and muscle contraction, malonyl-CoA production is inhibited through the AMPK pathway (51). However, it could be worthwhile to measure the AMPK and ACC levels at the gene expression level or cytoplasmic levels and examine their correlation with CPT-1 changes.
Our results demonstrated that the expression of HADHA in the gastrocnemius and soleus muscles increased in the resistance-grape oil and the HIIT- grape oil groups, respectively. In contrast, its expression in the HIIT and resistance groups had no significant changes. Inconsistent with our study, the results of Hoshino et al.'s research showed that the level of HADHA enzyme activity in both slow and fast fibers of the gastrocnemius muscle of male rats increased after four weeks of intense interval training (18). Christopher et al. found an increase in the activity of the HADHA enzyme in the skeletal muscles of athletes who performed six weeks of intense interval training of cycling on a carbometer (three days a week/10 repetitions with an intensity of 90% of the maximum oxygen consumption for 25 seconds and a 10-second rest) (52). In line with these results, our research showed that the HADHA gene expression in the soleus muscle of healthy male rats increased as a result of HIIT along with the consumption of grape seed oil. Due to its role in β-oxidation (53), the HADHA enzyme can promote the production of energy from free fatty acid sources. However, comparing HADHA gene expression and its activity needs further investigation.
5.1. Conclusions
HIIT, resistance training, and the consumption of grape seed oil may influence the gene expression of free fatty acid transporters in muscle skeletal cells and may improve fat metabolism. However, our results demonstrated that depending on the type of training (HIIT or resistance) and muscles (oxidative or glycolytic), the pattern of the expression of genes involved in the oxidation of free fatty acids (CD36, CPT-1 and HADHA) can be different.