1. Background
Clinically, ankylosing spondylitis (AS) is defined as a chronic inflammatory rheumatic disease, with main symptoms including pain in the back and peripheral joint stiffness with extra-articular manifestations (1) and severe and progressive spine involvement (2). Ankylosing spondylitis is more likely to affect men, which can be due to underdiagnosis or late recognition of this disease in women. Previous surveys are not powered enough to test similarities/differences across genders due to the enrolment imbalance; hence, it is a rare opportunity to pool data to address some of these problems concerning gender differences in AS (3). This pathology starts predominantly in young adults with chronic pain and disability, leading to a significant morbidity and mortality risk (4). Therapy options for AS patients include non-steroidal anti-inflammatory drugs (NSAIDs), non-biological disease-modifying antirheumatic drugs (DMARDs), such as sulfasalazine and methotrexate, and biological agents, such as tumor necrosis factor inhibitors (TNFi) and interleukin-17 (IL-17) antagonists (5). The clinical expression of some inflammatory pathologies, such as the manifestations, clinical evolution, and prognosis, depends on gender. Therefore, although different data have been published regarding the rate and frequency of radiological manifestations of AS in women, existing knowledge suggests stronger and more extensive radiographic changes in men (6). The expressed pathogenetic mechanism leading to gender-related disparities in AS manifestations is still unknown; it could be related to different haplotype combinations in the ankylosis homologous (ANKH) gene (7).
2. Objectives
The main purpose of this survey was to evaluate the disparities between men and women with AS in terms of clinical characteristics, structural damage, and treatment used in Algerian patients.
3. Methods
3.1. Patients
Our survey covered 292 patients diagnosed with AS at the Functional Rehabilitation Department of Hassani Abdelkader University Hospital of Sidi Bel Abbes region between 2018 and 2021.
3.2. Methods
We looked at various factors: Age, age at disease onset, disease duration, morning stiffness, medical history, articular and extra-articular injuries, laboratory data, favorable outcomes, HLAB27, disease activity markers, and treatment.
3.3. Statistical Analysis
Statistical analysis was done using SPSS 20. The categorical variables were tested using the chi-square and Fisher’s exact tests for continuous variables and the independent sample t-test for continuous variables. The level of significance was set at 5%.
4. Results
Among 292 AS patients, 166 women and 126 men were enrolled. The mean age at disease onset was 31.69 ± 10.675 and 30.38 ± 10.250 years in females and males, respectively. There was a significant difference between females and males concerning disease duration (P = 0.287) and AS morning stiffness duration (26.08 ± 26.977 vs. 23.80 ± 26.529, respectively; P = 0.911). Elevated erythrocyte sedimentation rate (ESR), positive C-reactive protein (CRP), and positive HLAB27 were respectively more observed in women 83.8%, 59.9%, and 71.9% than in men. Most male patients were smokers compared to females (21.6% vs. 0.6%; P < 0.0001) (Table 1).
| Characteristics | Men (n = 126) | Women (n = 166) | P-Value |
|---|---|---|---|
| Age | 38.03 ± 11.981 | 38.44 ± 12.248 | 0.478 |
| Age at disease onset | 30.38 ± 10.250 | 31.69 ± 10.675 | 0.499 |
| Disease duration (y) | 7.28 ± 4.867 | 6.74 ± 3.584 | 0.287 |
| Duration of morning stiffness (minutes) | 23.80 ± 26.529 | 26.08 ± 26.977 | 0.911 |
| Laboratory data | |||
| ESR titer (mm/h) | 41.516 ± 26.413 | 48.684 ± 30.091 | 0.072 |
| Elevated ESR | 102 (81.6) | 140 (83.8) | 0.616 |
| CRP titer (mg/L) | 20.219 ± 22.336 | 19.320 ± 23.623 | 0.547 |
| Positive CRP | 67 (53.6) | 100 (59.9) | 0.283 |
| HLAB27 | 87 (69.6) | 120 (71.9) | 0.675 |
| Smoking | 27 (21.6) | 1 (0.6) | < 0.0001 b |
Demographic and Laboratory Data of Patients Based on Gender a
Cervical damage was more reported in men but with no significant difference (44% vs. 37.1%, P = 0.236), and lumbar was more affected in women with a significant difference (100% vs. 39.2%, P < 0.0001). Besides, radiological joint damage was more noted in the men, with a significant difference between the two groups in knees and hips (P < 0.0001 for both). The scores of bath ankylosing spondylitis disease activity index (BASDAI) and (ankylosing spondylitis disease activity score (ASDAS)-CRP) were significantly higher in men than in women (3.758 ± 2.309 vs. 2.334 ± 1.232 and 3.071 ± 1.222 vs. 2.301 ± 1.266; P < 0.0001 and P = 0.003, respectively). The number of men in the severe stage of the disease (52.8% vs. 41.9%) was significantly more than women (P < 0.0001) (Table 2).
| Characteristics | Women (n = 166) | Men (n = 126) | P-Value |
|---|---|---|---|
| Spine damage | |||
| Cervical | 62 (37.1) | 55 (44) | 0.236 |
| Lumbar | 167 (100) | 49 (39.2) | < 0.0001 b |
| Radiologic joint damage | |||
| Knees | 6 (3.6) | 33 (26.4) | < 0.0001 b |
| Hips | 5 (3) | 87 (69.6) | < 0.0001 b |
| Disease activity indices | |||
| BASDAI | 2.334 ± 1.232 | 3.758 ± 2.309 | < 0.0001 b |
| ASDAS-CRP | 2.301 ± 1.266 | 3.071 ± 1.222 | 0.003 b |
| Disease activity | < 0.0001 b | ||
| Inactive | 51 (30.5) | 14 (11.2) | |
| Moderate | 40 (24) | 22 (17.6) | |
| High | 70 (41.9) | 66 (52.8) | |
| Very high | 6 (3.6) | 23 (18.4) |
Radiologic Data of Ankylosing Spondylitis Patients Based on Gender a
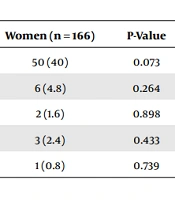
Uveitis and psoriasis were the most recorded comorbidities shared between the two groups (Table 3). Also, 29.9% of men had uveitis vs. 40% in women, 2.4% had psoriasis vs. 4.8% in women, 1.8% had Crohn’s disease vs. 1.6% in women, 1.2% had diabetes vs. 2.4% in women, and 1.2% had renal failure vs. 0.8% in women. Sulfasalazine, Humira, and Remicade treatments were significantly associated with sex P = 0.027, P = 0.002, and P = 0.014, respectively (Table 4).
5. Discussion
Our study is the first report in the west of Algeria on the differences between men and women regarding AS characteristics.
Previously, AS was recorded more in male than female patients (3). In our study, 166 cases (56.84 %) were women, and 126 (43.15%) were men. Gran and Skomsvoll found that the number of female patients was more than men (33%) in most reports on AS (8). The mean age of AS patients was 38.44 ± 12.248 years in women vs. 38.03 ± 11.98 years in men with no significant association (P = 0.478), which is consistent with reports by Jung et al. (P = not significant (NS)) (9) and Shahlaee et al. (P = 0.19) (10). Looking at the difference between the two studied groups, we did not find an association between age at disease onset and sex (P = 0.499), which matches with the results of Feldtkeller et al. (P = NS) (11), Ibn Yacoub et al. (P = 0.374) (12), and Webers et al. (P = 0.92) (13). However, Chen et al. (P = 0.001) (1), Jung et al. (P = 0.015) (9), Shahlaee et al. (P = 0.041) (10), Ciurea et al. (P < 0.001) (14), Kim and Kim (P = 0.05) (15), Lee et al. (P = 0.03) (16), and Qian et al. (P < 0.001) (17) reported a significant association between age at disease onset and sex. We did not find an association between disease duration and sex (P = 0.287), which is consistent with some reports (10, 12, 15-18), while some researchers found a significant association (1, 3, 9, 14). First, we propose that women have a younger age at onset, which is why they suffer longer from AS than men. Concerning morning stiffness, our results and those of Ibn Yacoub et al. were contradictory (26.08 ± 26.977 vs. 23.80 ± 26.529; P = 0.911, 43.6 ± 26.8 vs. 64.7 ± 25.3; P = 0.007) (12), respectively.
Sex was positively associated with ESR titer (mm/h) in the studies by van der Horst-Bruinsma et al. (P < 0.001) (3), Webers et al. (P = 0.04) (13), Qian et al. (P < 0.001) (17), and Shahlaee et al. (P < 0.001) (10). Nonetheless, some researchers did not find this association, such as Ibn Yacoub et al. (12), Webers et al. (13), Qian et al. (17), and Shahlaee et al. (P = 0.719) (10), which is consistent with our results (P = 0.072). Furthermore, no significant association has been reported between CRP titer (mg/L) and gender (P = 0.547) (12). Positive CRP value was more noted in women than in men with no signification (P = 0.283), which concords with some results (13, 17), such as Benhamou et al., who reported higher CRP values in women than men (19).
Positive HLA-B27 was more noted in women (71.9%) than in men (69.6%). No correlation was found between HLA-B27 and gender (P = 0.675), which is similar to the findings of Webers et al. (P = 0.10) (13), Kim and Kim (P = NS) in the Korean population (15), and Lee et al. (P = 0.22) (16). However, it is not in accordance with those previously published (3, 9, 14, 17), which can be explained by racial or geographic predisposition (17). HLA-B27 may be less common among Algerian women with AS (10).
Lumbar joint was significantly more affected in female patients (167, 100%), which is in accordance with the results of Jung et al. and Shahlaee et al. (P = NS and P = 0.063, respectively) (9, 10). No differences in cervical symptoms were reported in our study (P = 0.236) and studies by Jung et al. (P = NS) (9) and Shahlaee et al. (23.9%) (P = 0.226) (10). According to Gran et al., no differences in spinal mobility existed between men and women with AS (20). Some authors found that AS course is more benign, and the radiologic progression is slower in women (15, 16). Also, Lee et al. (16) found that women had less spinal mobility impairment, while men were found to have higher spinal involvement and bamboo spine. Men tend to have more severe radiographic damage, and syndesmophytes, inflammation, and mechanical factors may contribute to radiographic damage (8, 11, 13, 16, 21-24). Qian et al. showed that men had higher CRP levels and hence a higher frequency of ESR positivity, a sign of more severe inflammation, and uncontrolled inflammation confers more rapid radiographic progression (17).
We found that women significantly suffered more in their peripheral joints than men (knees (P < 0.0001): 26.4% vs. 3.6%; hips: 69.6% vs. 3%, respectively), which is in line with the study by Jung et al. (P < 0.001) (9). We also recorded an association between hip arthritis and male sex, which confirms the report by Shahlaee et al. (P < 0.0001) (10). Recently, Kim and Kim noted a significantly higher peripheral involvement in men and axial involvement in women (15). Some authors reported that women with AS presenting primarily peripheral arthritis are often considered for having another rheumatic disease diagnosis before AS diagnosis (12, 16).
In our population, the disease activity indices were higher in men than in women; we found a positive association between BASDAI and sex, as reported by Landi et al. (P < 0.001) (18), van der Horst-Bruinsma et al. (P < 0.001) (3), and Ibn Yacoub et al. (P = 0.012) (12). In contrast, this association was not significant in other studies (9, 10, 13, 17). Webers et al. (13) did not report an association between ASDAS-CRP and sex (P = 0.74), whereas, in our research, this relationship was significant (P = 0.003). There is a discordance in the literature, mainly concerning women having significantly lower educational levels than men, particularly in rural areas. In chronic rheumatic diseases, patients with higher academic levels had more severe disease outcomes with altered health status (13, 25-27).
Other papers have stated an association between gender and patient comorbidities, including studies by Shahlaee et al. (10), Webers et al. (13), Kim and Kim (15), Landi et al. (18), and Qian et al. (17) as confirmed by our findings. Likewise, female patients with psoriatic spondylitis experienced better spinal mobility and lower radiologic damage; in our study, 6 women (4.8%) vs. 4 men (2.4%) had psoriasis. Most studies have found no differences in uveitis between both genders, but uveitis in females was more noticed in a few studies, such as Kim and Kim (15, 20, 28), as reported in our results (4.8% vs. 2.4%; P < 0.0001)). The stated pathogenetic mechanism that results in gender-associated disparities concerning AS manifestations is still ambiguous. Also, several haplotype combinations in the ankylosis homolog (ANKH) gene were noticed in males and females with AS; more genetic investigations might identify other genes contributing to observed ethnic and gender disparities and AS severity (7, 9).
Furthermore, we demonstrated a relationship between sex and sulphasalazine treatment (P = 0.027), which is in accordance with the results of Chen et al. (P = 0.062) (1), Shahlaee et al. (P = 0.835) (10), and Lee et al. (P = 0.000) (16). Similar results were found for Remicade treatment (10, 16). Concerning other drugs, we did not find a relationship with gender, which concords with many studies (1, 10, 13, 16). Women in the cohort of Lee et al. did not have higher rates of sulfasalazine, methotrexate, and intra-articular steroid use.
Moreover, the frequency of sulfasalazine and steroid use was more than in the PSOAS cohort, possibly due to a recall bias in the PSOAS cohort or ethnic disparities (1, 16). In our study, women were treated with Humira more than men (88.6% vs. 74.7%), which indicates why they suffer more from their lumbar.
Longitudinal studies are necessary to confirm these results and identify gender-specific AS parameters. The diagnosis and therapeutic management of this condition may be aided by understanding connections between gender and disease characteristics in the context of our inquiry, where many demographic and socioeconomic factors might influence the disease outcome.
5.1. Conclusions
In our study, men expressed a higher rate of accelerated ESR and positive CRP, and positive HLAB27, and the spine of women was much more affected than men. It is crucial to conduct more research on gender disparities for a healthy Algerian population.