1. Background
Pulse oximeters alert healthcare providers to blood oxygen saturation drops. It is a non-critical device that comes into contact with intact skin and does not touch patients' mucosal surfaces. However, patients admitted to intensive care units for a long time develop low skin integrity and are more susceptible to hospital-acquired infections (HAIs) caused by abrasions like pressure sores and pre- or post-operative trauma caused by improperly applied sensors (1).
Pseudomonas, Escherichia coli, Klebsiella, Methicillin-resistant staphylococcus aureus (MRSA), Acinetobacter, and Clostridium difficile contaminate hospitals, especially critical care units (2). Humidity, low temperature, multi-drug resistance, and other environmental factors increase microbe survival, which leads to HAIs. Since patients and staff shed these organisms, surfaces, and equipment like pulse oximeters, stethoscopes, blood pressure cuffs, and others are frequently contaminated (3, 4).
Manual disinfection approaches are effective when used consistently (1). Reusable pulse oximeter probes are noncritical equipment, requiring only low-level disinfection between patients as long as the probe is used on intact skin and not soiled with blood or other bodily fluids (5). Patients' medical devices are sterilized with 70% diluted alcohol or sodium hypochlorite. However, alcohol destroys spores better than sodium hypochlorite (3). The pulse oximeter, an essential medical instrument used in Pakistani hospitals, has no published information on sensor contamination and decontamination.
2. Objectives
This study aims to identify microbial contamination on intensive care unit pulse oximeter sensors in public and private hospitals. It compares pulse oximeter sensor cleaning with alcohol and sodium hypochlorite.
3. Methods
A cross-sectional study was conducted in three private and two public sector tertiary care hospitals in Karachi with ICU facilities. Sixty-four functional pulse oximeters were selected for the study and equally divided into pre- and post-decontamination groups. Each sample was single-blind coded, and 14 blank samples were included. Disposable, defective, or broken pulse oximeters used outside the ICU or not attached to patients for more than 24 hours were excluded. Ethical approval and consent were obtained from relevant authorities, and the confidentiality of hospitals was maintained.
After taking all aseptic measures, each upper and lower surface of the finger sensor pad's inner side was sampled using the surface sample technique at 1.5 cm × 3 cm. The swab was moistened with sterile 0.9% Normal Saline and was used to remove organic debris. After a baseline sample, the samples were randomly assigned and disinfected with freshly prepared 70% isopropyl alcohol or 10% sodium hypochlorite. The swabs were placed in sterile transport gel tubes with a code number given for laboratory blinding. All aseptic measures were taken to avoid cross-contamination during the sampling process.
Samples were collected in an ice box and taken to the laboratory for analysis within 45 to 90 minutes. Blood agar and MacConkey agar were used to inoculate the samples, which were incubated for 24 hours at 37°C. Microbial growth was examined, and gram staining and biochemical tests were performed for organism identification. The Miles and Misra technique determined the microbial load, and colonies were counted using a colony counter as CFU/mL. Methicillin-resistant staphylococcus aureus and MSSA were identified using the Cefoxitin disc screen test.
SPSS version 22 was used for data analysis, which included the hospital sector, microbes before and after disinfection, and disinfectants used as variables. McNemar's test was used to determine the association between qualitative variables, with a P-value < 0.05 considered statistically significant.
4. Results and Discussion
Pulse oximeters are noncritical medical devices that touch intact skin but not mucous membranes. Although such devices are virtually risk-free, oximetry sensors or dry or cracked locations increase microbe transmission. The first Pakistani study examined microbiological contamination and disinfection efficacy in pulse oximeters used in public and private hospitals.
Of the 68 pulse oximeters screened, 25 (36.76%) were contaminated by microorganisms; 13 and 12 samples showed microbial growth in public and private sector hospitals, respectively. Manual disinfection reduced the microbial load, preventing organism transfer to workers and patients. Despite using alcohol as a disinfectant, US research reported microbial contamination on reusable pulse oximeters (6). It shows that cleaning is crucial and disinfectants don't always kill bacteria. However, noncritical surfaces that touch patients or healthcare personnel should be cleaned and disinfected daily, and shared equipment should be disinfected between patients and at terminal cleaning (1). The US Centers for Disease Control and Prevention (CDC) recommend similar protocols for disinfecting noncritical environmental surfaces and patient care equipment (7).
Both private and public hospitals had lower baseline organism counts. Hospital disinfection routines, patients admitted from a socioeconomic class with good hygiene (reducing the chance of acquiring virulent organisms), and strict cross-contamination prevention measures like separate gloves for each sample may explain this. Our study's baseline organism differences may be due to pulse oximeters' narrow surface area, which is unlikely to produce high baseline counts. Despite training in sample collection, human error can affect the results.
The break-up spectrum of bacterial isolates from the devices reported Staphylococcus species as the most common bacterial isolate (10 samples), followed by MSSA (5 samples), MRSA (6 samples), and Pseudomonas aeruginosa (4 samples).
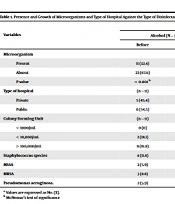
The maximum microbial load was in colony-forming units > 100,000/mL in public and private sector hospitals (15 samples). Both Alcohol and Sodium Hypochlorite used as a disinfectant were statistically effective (P < 0.05) in reducing the microorganism in both private and public sector hospitals. Alcohol was an effective disinfectant than Sodium hypochlorite for all reported microorganisms. (Table 1).
| Variables | Type of Disinfectant | |||
|---|---|---|---|---|
| Alcohol (N = 34) | Sodium Hypochlorite (N = 34) | |||
| Before | After | Before | After | |
| Microorganism | ||||
| Present | 11 (32.4) | 03 (8.8) | 14 (41.2) | 03 (8.8) |
| Absent | 23 (67.6) | 31 (91.2) | 20 (58.8) | 31 (91.2) |
| P-value | < 0.001 b | < 0.001 b | ||
| Type of hospital | (n = 11) | (n = 03) | (n = 14) | (n = 03) |
| Private | 5 (45.4) | 2 (66.6) | 7 (35.0) | 2 (66.6) |
| Public | 6 (54.5) | 1 (33.3) | 7 (35.0) | 1 (33.3) |
| Colony forming unit | (n = 11) | (n = 03) | (n = 14) | (n = 03) |
| < 1000/mL | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| < 10,000/mL | 2 (18.1) | 1 (33.3) | 8 (57.1) | 2 (66.6) |
| > 100,000/mL | 9 (81.8) | 2 (66.6) | 6 (42.8) | 1 (33.3) |
| Staphylococcus species | 4 (11.8) | 1 (2.9) | 6 (17.6) | 2 (5.9) |
| MSSA | 2 (5.9) | 1 (2.9) | 3 (8.8) | 0 (0) |
| MRSA | 3 (8.8) | 0 (0) | 3 (8.8) | 0 (0) |
| Pseudomonas aeruginosa | 2 (5.9) | 1 (2.9) | 2 (5.9) | 1 (2.9) |
a Values are expressed as No. (%).
b McNemar's test of significance
Alcohol disinfection significantly outperforms sodium hypochlorite in our study. According to Nandy et al., commercial alcohol wipes disinfect better than sodium hypochlorite, which is sporostatic rather than sporicidal (8, 9). Sodium hypochlorite spraying also reduced contamination by 50% in another study (10).
One limitation of our study is that the study did not directly investigate the clinical outcomes of patients who used contaminated devices compared to non-contaminated ones. This approach could have provided more robust evidence regarding contamination's impact on patient health. Other limitations include limited resources, a short data collection time, and some hospitals' refusal to collect samples.
Future studies should consider incorporating a clinical component to further elucidate the significance of contamination in pulse oximetry probes and its potential consequences.
4.1. Conclusions
This study showed that noncritical medical devices like pulse oximeter sensors could harbor potentially contagious infections like antibiotic-resistant bacteria. Alcohol reduced bacterial load better than another disinfectant. With better cleaning methods and patient-specific pulse oximetry sensors, alcohol reduces the risk of nosocomial infections and is cost-effective.