Introduction
Researches on human and animal models have revealed many molecular changes related to wound healing of diabetes mellitus patients [1]. Delay in wound healing of these patients is related to delay in cellular diapedes, occurrence of granulated tissue, fibroplasia, malorganization of collagen fibers, decrease in local blood flows, increase in blood viscosity and decrease in angiogenesis [2].
Mast cells could be seen affluent in some fibrotic disorders [3]. Uprising findings show affecting of the mast cells on wound healing [4]. Mast cells have many cytokines that could be involve in remodeling of extracellular matrix during wound healing process [2]. Mast cells are cooperating in at least inflammation, angiogenesis, and absorption of extracellular matrix and remodeling of extracellular matrix during wound healing process [5, 6]. Tissue fibrosis is characterized by substation of normal components of tissue structure with an intense fibrotic and unworkable scar tissue and in these conditions the amount of mast cells increases [7].
A few studies have been shown that pentoxifylline (PTX) is effective for the treatment of diseases related to tissue fibrotic such as chorionic wound healing, necrotic wounds in the diabetic veins (venous leg ulcer), pulmonary fibrotic inflammation, fibrosis of interensitial tissue of the kidney, phlegmona of the foot, and sarcoidosis [8-11]. Pentoxifylline is a selective drug for the dilatation of the vessels in the limbs, brain and retina. Dilatation of the vessels is due to suppressing phosphodiesterase enzyme and increasing concentration of cAMP in the smooth muscles of the vessels. PTX increases diapedes of red cells among the wall of capillaries by decreasing the viscosity of these cells along with increasing fluidity of the cell membrane. PTX improves microcirculation of the vessels, blocks the action of neutrophils and increases or decreases production of some cytokines like TNF-α [12].
There are a few researches related to the study of the effect of PTX for maturity and degranulation of mast cells in skin wounds and present study was conducted based on active and undeniable effects of mast cells and that were stated about the effect of PTX on inflammation processes to study the effect of PTX on maturation of mast cells and to evaluate their granules and also to show the efficacy of PTX on skin wound healing of normoglycemic and diabetic rats.
Materials and Methods
This study was approved by the Ethical Committee for animal use of the Shahid Beheshty University of Medical Sciences. In this study 48 male wistar rats weighting 250-350 g were obtained from Pasteur Institute of Iran and were maintained in animal house of faculty of medicine of Shahid Beheshty University, one per age with free access to food and water in a room with controlled humidity and temperature 22-24°C on a 12 h light/dark cycle and housed one per cage. The methods of the present study were approved by the Ethical Committee for animal use of the Shahid BeheshtyUniversity of Medical Sciences. Diabetic animals (N=12) were randomly selected and for inducing diabetes, the food was uptake from the cages 12 h prior the experiments and then administrated with intraperitoneal injection of streptozotycine (55 mg/kg; Zanosar Pharmacia & Upjohn Co, Kalamazoo, Ml 49001, USA) dissolved in distilled water. Seven days later, blood was collected from the tail of animals and the amount of serum glucose was determined by the related device (Supreme Petit, Hypoguard LTD with test strips of Supreme 50 test code 56) and if the serum level of glucose was higher than 300 mg/100 mL, the animal was considered for diabetic group (N=12 for experimental and control groups). If the animal did not show the sign of diabetes, only another injection was performed and another negative result brought the animal to be omitted from the study and substituted by the second rat. During a month later, the serum level of glucose was evaluated in each three days and if the level was not lower than the 300 mg/100 mL, the animal maintained in the study. Control group (normal and diabetic) were received distilled water besides PTX. Normal group of rats (N=12), also selected randomly and housed one per cage and glucose serum level was analysis for approving the rats were normoglycemic. PTX was injected interaperitoneally (25 mg/kg) in two times per day since 4 days prior wound incidence up to the end of the experiments (7 days after surgery) [13].
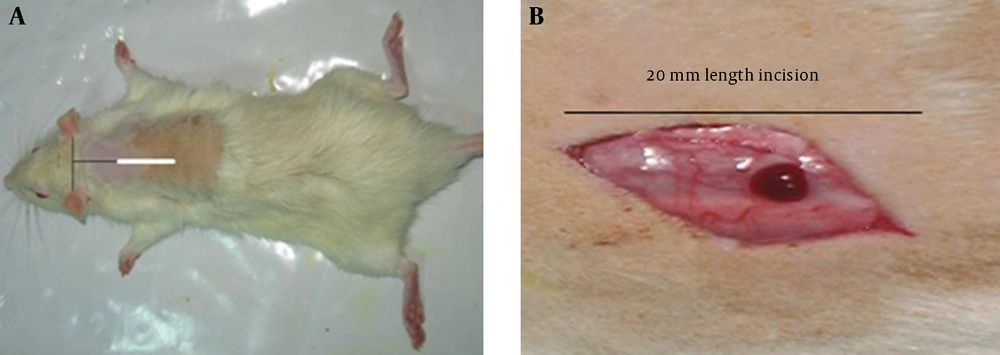
To induce wound in the hole skin thickness, at first the rats (24 rat in diabetic and normoglycemic groups respectively) were anesthetized with intraperitoneal injection of ketamine (50 mg/kg) and rampon (5 mg/kg) and the backs of the rats were shaved and a full-thickness incision wound was made to the level of the panniculuscarnosus muscle [13, 14]. The wounds were not sutured or covered and left to be healed by second intention. Five rats were died during anesthesia and post wounding period and were replaced by new ones.
To microscopically evaluation of the wound healing process and maturation of the mast cells in days 3 and 7, the rats were sacrificed in desiccators jar and complete area of wound was excised (including incision area and adjacent normal skin). Tissues were fixed in formalin 10% (pH=6.8) and labeled by coding. After fixation, tissue processing and blocking the tissues with paraffin was performed. The 6 µm sections were stained with toloidene blue (10%) and the pictures were captured by light microscope (Nikon, Japan, 8 Mega Pixel) (10×100) with emersion oil. The width of the wounds were 3.15 mm2 for all the groups similar to 150 fields at the border of incidence, granulation tissue, and regenerating collagen zone were captured randomly. The pictures were evaluated by stereological methods [15].
Stereological method applied in this study: In present study, from each wound healing tissue in the days 3 and 7, 15 double serial sections were prepared named as reference and look up plans with 5 section separations and also 30 section separations between each couple of selected sections. Counting of mast cells was done based on random accordance of specific grades for counting tissue particles [13, 14]. In this study, the rats were divided into two normal and diabetic groups then each of these groups subdivided into two experimental and control groups. If we consider that the length of each sampling tissue (skin wound) is 5 mm (500 µm) and 6 µm sections are prepared, there is needed 833 sections for each subject and for all 24 subject is 19,992 (according stereological method the length of section must be at least 30% of the sampling tissue) and bodily that is seem not possible for preparing these amounts of sections. In this condition, to achieve relative accurate statistical data of the number of blood vessels or distribution of specific cells, there is needed for applying stereological method. In this method, there is necessary for knowing the dimensions and characteristics of the studied subjects. For example, to evaluate distribution of the mast cells, we should know the length of the cells (the length of each mast cell is 20-30 µm) then to gain relative accurate estimation, it should be needed to prepare two near there is needed 6 µm diameter reference and look up sections (a section with 30% of the length of 20-30 µm mast cells).
Data processed using SPSS-16, by K-S, Levens test, Student sample t-test, one-way ANOVA analysis tests. The results were determined based on mean±standard error of mean and significance was set up at p<0.05.
Results
Weight of most of the diabetic rats and a few of normal rats decreased but these data were not significant. The amount of glucose was higher than 300 mg/ 100 mL in diabetic rats. The differences in the rate of glucose in the groups and between groups did not shown any significant differences.
| Type one of mast cells | Type two of mast cells | Type three of mast cells | Summations | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | Group A | Group B | Group C | Group D | Group A | Group B | Group C | Group D | Group A | Group B | Group C | Group D | |
| 14.3 | 15 | 12.2 | 14.2 | 20.9 | 19.2 | 24 | 21.6 | 7.2 | 8.1 | 18.5 | 19.5 | 14.3 | 14.1 | 19 | 18.7 | |
| 16.8 | 14.7 | 13.5 | 16.9 | 27 | 17.6 | 30 | 27.3 | 5.9 | 14.8 | 18.2 | 22 | 16.8 | 20.1 | 20 | 20.7 | |
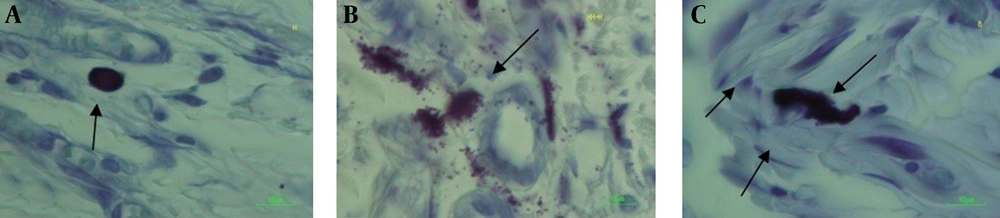
The basis for characterization and categorizing of mast cells for counting is shown in figure 2. The mean number of mast cells (1+2+3) in each group and for each day of post surgery in microscopically evaluation in 3.15 mm2 of width of the wounds in the days 7and 3 with 10×100 magnification and after histological and stereological evaluations are shown in table 1.
In the day three, the mean number of type 3 of mast cells between normoglycemic and diabetic rats was shown significant statistical difference (p=0.0001).
In the day 7 after surgery as that shown in table 1: in the normal part of the study, there was a significant difference in the number of type 2 of mast cells between PTX and control groups (p=0.0001). In the day 7 in normal part of the study, there was also significant differences in the mean number of type 3 of mast cells between PTX and control groups (p=0.0001).
Discussion
In present study, streptozodicine was administrated to rats for induction of diabetes and although this type of diabetes has no prolonged clinical features as that for human is a suitable model for the study of diabetic wound [16]. Considering the mean number of mast cells, it seems that the type 2 of mast cells in all PTX treated groups was significantly increased in the day 7 after surgery. Although the mean number of type 3 of mast cells in DB+PTX group in comparing with control ones in the day 7 after surgery was lower, this decrease was not significant. However, considering the rate of decrease in type 3 of mast cells in the days 3 and 7 in DB+PTX group in comparison with DB+DW it seems that PTZ could delay the maturation process of type 2 to 3 of mast cells. The data showed that the type 3 of mast cells was reduced in NG+PTX group in comparison with control one and in other words, PTX caused a delay for maturation of the type 2 to 3 of mast cells. According these data, PTX could be considered as a modulator of maturation of type 2 to type 3 of mast cells. Many studies showed that reduction of the components of inflammation procedure such as degranulation of mast cells has positive effect on wound healing [4, 17]. In diabetes mellitus, because of high level of serum glucose, decrease in the rate of replication of the cells exactly in fibroblasts induces that causes deficient in collagen synthesis during wound healing [9, 18-22]. In present study it also showed that delay in wound healing process in diabetic group in comparison with control group was occurred in parallel with increasing in the number of type 3 of mast cells. But considering timing, it seems maturation of type 2 to 3 of mast cells in all PTX treated groups occurred and this data showed that maturation of mast cells in DB+PTX has a similar pattern as that for normal group. In other words, if the timing of the experiment were longer and the diabetic rats administrated longer with PTX, process of maturation of type 2 to 3 of mast cells was shown more similarity between diabetic and normoglycemic groups.
The study showed although the number of total mast cells in PTX groups was lower this decrease was not statistically significant. Recent studies showed that mast cells are involved for creating scar tissue during wound healing process [19]. Thus, reduction of the number of mast cells that was similar to normal rats may consider for clinical application of PTX for omitting scar tissue of wounds and this effect of PTX could be resulted by decreasing in the number of fibroblasts [19-22]. Some studies showed that IL-1α, IL-9, prostaglandin E2, monocyte chemotactic protein-1 are factors that could increase accumulation of mast cells by a mechanism related to fibroblasts [23] and because PTX has blocking effect on fibroblasts, this efficacy could indirectly suppress occurrence of scar. On the other hand, recent studies are shown that PTX are able to decrease tissue damage regarding to inflammatory factors of neutrophils such as IL-1, TNF-α and because IL-1 is a inducer factor for progression of mast cells, PTX by blocking IL-1 suppress the maturation of mast cells [24].
It also been shown that during normal wound healing, there are a few changes in the amount of monocyte chemotactic protein-1 (MCP-1) along with degranulation of mast cells [10, 20]. Recent data showed that PTX was decreased the expression of MCP-1 in a dose dependent manner [25, 26] that finally caused the decreasing in the progression of inflammation, increase in the velocity of healing process and blocking in scar formation. The study adds that PTX does these effects by increasing in the number of type 2 of mast cells and also recucting in the maturation process of type 2 to 3 of mast cells.
Further investigations need for other probable mechanisms that could affect delay of maturation of type 2 to 3 of mast cells derived by PTX. Here it is suggested that by conducting a similar study, different doses of PTX with longer follow up could evaluate accurately the effect of PTX on maturation of mast cells.