Introduction
In the last decades expanding telecommunication technologies have become one of the most important source of non-ionizing high-frequency electromagnetic fields (EMF). Exposure to radio frequency fields emitted by mobile telephones (which operate between 400 and 2,000 MHz frequency bands) and their base transceiver station (BTS) has become a major concern to the public regarding the possible adverse effect of high-frequency EMF on human health.
In the previous investigations it was reported that non-thermal effects of the radio frequency wave (RFW) exposure is able to induce several changes in the DNA level [1], alteration of heat shock proteins [2], and increase permeability of the human blood-brain barrier [3]. Reports of potential adverse effects of RFW on the brain [4], hematological parameters and bone marrow [5] and endocrine system [6] in human and animals are widely documented in the literature. Erythrocytes are especially vulnerable to oxidative stress because of 1- the pentose phosphate pathway 2- active metal protein (hemoglobin), which functions as an oxidase and peroxidase, 3- membrane proteins and unsaturated fatty acids which can be oxygenated, and 4- higher tension oxygen than any other cells in the body, with the exception of lung cells [7]. Oxidative damage in the erythrocytes can lead to loss of cell function. Reducing the activity of superoxide dismutase in red blood cell causes oxidative stress. The results of this phenomenon lead to loss of symmetrical structure of cell membrane lipids, loss of flexibility, impaired water and ions exchange and ultimately cell swelling [8]. Decrease in enzyme activity are involve oxidation Fe2+ to Fe3+ of hemoglobin will decrease O2 transportation following the disturbances, anemia occurs with the formation Heinz body [9, 10].
L-ascorbic acid is a 6-carbon lactone ring structure with 2, 3-enediol moiety. The antioxidant activity of ascorbic acid comes from 2, 3-enediol [11]. Vitamin C is a powerful antioxidant, acts outside and within the cell and provides a protective effect against several diseases including oxidative imbalances arising from various causes in the erythrocyte and other tissues [12-14], also, vitamin C acts as a pro-oxidant, depending on the environment in which the molecule is present. Intracellular vitamin C concentrations in the low millimolar range (much higher than that in plasma) seem to be necessary to support its role as an antioxidant.
The aim of the present study is to investigate the effects of 900 MHz radio waves on antioxidant enzymes activity and malondialdehyde (MDA) levels in the rat erythrocyte and to evaluate the protective effects of vitamin C in these cells.
Materials and Methods
Animal Experiments
All investigations were conducted in accordance with the "Guiding Principles for the Care and Use of Research Animals" approved by Shiraz University. Thirty-two adult male Sprague-Dawley rats (220±15 g) colony-bred in the Animal House Center, Shiraz, Iran, were housed (8 rats per cage) in the animal room under controlled lighting (12 h light: 12 h darkness) and temperature (20±2ºC) conditions and had free access to a pelleted food (formulated and made by Javaneh Khorasan Company, Iran) and tap water. All of the experimental procedures were carried out between 09:00 and 13:00.
Radio Frequency Signal Generator
The signal generator for producing a 900 MHz signal was made in the Department of Electrical Engineering, Shiraz University, and the output was monitored by a spectrum analyzer (FSH6, from Rohde and Schwarz, Germany) to ensure the correct forward power from the custom-designed mobile base stations on the animals exposed. The power of the BTS antenna at minimum distance from their installation site to a citizen’s residence (17 m) was measured via a probe connected to the spectrum analyzer. The power level reading was -75 db. Based on our calculation and evaluation, the signal generator could radiate the same power level at a 5 m distance. Hence, the test group and treated group were placed 5 m from the signal generator.
Blood and Hemoglobin Preparation
On the final day of exposure to RFW, the animal of all groups were killed under ether anesthesia, by whole blood collection through heart puncture. The heparinized blood was centrifuged to remove plasma components. The packed red cells were washed three times in an isotonic saline solution (0.9% NaCl) and red cells were osmetically lysed with cold distilled water (2 mL). Hemoglobin (Hb) was measured using cyanmethemoglobin method [8].
Superoxide dismutase (SOD) assay
Total SOD activity was evaluated with SOD detection RANSOD kit (Randox lab. Crumlin United Kingdom) according to the manufacturer instructions. The role of SOD is to accelerate the dismutation of the toxic superoxide produced during oxidative energy processes to hydrogen peroxide and molecular oxygen. This method employs xanthine and xanthine oxidase (XOD) to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. The SOD activity is then measured by the degree of inhibition of this reaction. One unit of SOD is that which causes 50% inhibition of the rate of reduction of INT under the conditions of the assay. SOD levels were recorded at 505 nm and through a standard curve and expressed unit per gram of hemoglobin (U/g Hb).
Glutathione peroxidase (GPx) assay
The activity of GPx was evaluated with GPx detection RANSEL kit (Randox lab. Crumlin United Kingdom) according to the manufacturer instructions. GPx catalyze the oxidation of glutathione (GSH) by cumene hydroperoxide. In the presence of glutathione reductase (GR) and NADPH, the oxidized glutathione (GSSG) is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance at 340 nm against blank was measured spectrophotometrically. One unit (U) of GPx activity was defined as the amount of enzyme that converts 1 μmol of NADPH to NADP+per minute. The GPx activity was expressed as unit per gram of hemoglobin (U/g Hb).
Catalase (CAT) assay: Tissue catalase activity was assayed spectrophotometrically by monitoring the decomposition of H2O2 using the procedure of Aebi [15]. Briefly, 0.5 mL of 30 mM/L H2O2 solution in 50 mM/L phosphate buffer (pH=7.0), 1 mL of 1:10 diluted erythrocyte lysates was added and the consumption of H2O2 was followed spectrophotometrically at 240 nm for 2 min at 25ºC. The molar extinction coefficient was 43.6 L/M per cm for H2O2. Catalase activity was expressed as the unit that is defined as µM H2O2 consumed/min/g hemoglobin.
Measurement of lipid peroxidation (MDA):
To evaluate lipid peroxidation in blood a modified HPLC method was used which is based on the reaction of malondialdehyde (MDA) with thiobarbituric acid (TBA) to form a coloured MDA-TBA adduct [16]. Briefly, 0.5 mL blood supernatant was added to 2 mL TBA reagent containing 0.375% TBA, 15% trichloroacetic acid and 0.25 M/L HCl. the mixture was immediately heated (60 min at 95°C) and cooled with running water, and thereafter butanol–pyridine (15:1, v/v) (1 mL) was added and the final volume was adjusted to 2 mL with distilled water. After vigorous mixing, the organic layer was separated by centrifugation (16000 g, 3 min, at room temperature). The supernatant was analyzed on a UV-visible spectrophotometer fitted with an 80 µL flow cell [17, 18]. The absorbance was measured at 532 nm (the mobile phase consisted of 300 mL/L methanol in 50 mM KH2PO4, pH: 7.0). 1, 1, 3, 3-tetraethoxypropane was used as a standard, and MDA-TBA reactive substances values were expressed as Unit per gram of hemoglobin (U/g Hb). The HPLC system consisted of a solvent delivery pump (JASCO 980-PU, Tokyo, Japan), a reversed-phase column (Luna C18, 250 mm×4.6 mm, Phenomenex, CA, USA), and a UV-Vis detector (Jasco, UV-975, Tokyo, Japan) operated at 532 nm.
Statistical Analysis: The results were expressed as means±SEM. All data were done with the Statistical Package for Social Sciences (SPSS-16.0 for windows). The results were analyzed using one way analysis of variance (ANOVA) followed by Tukey test for comparison between different treatment groups. Statistical significance was set at p<0.05.
Results
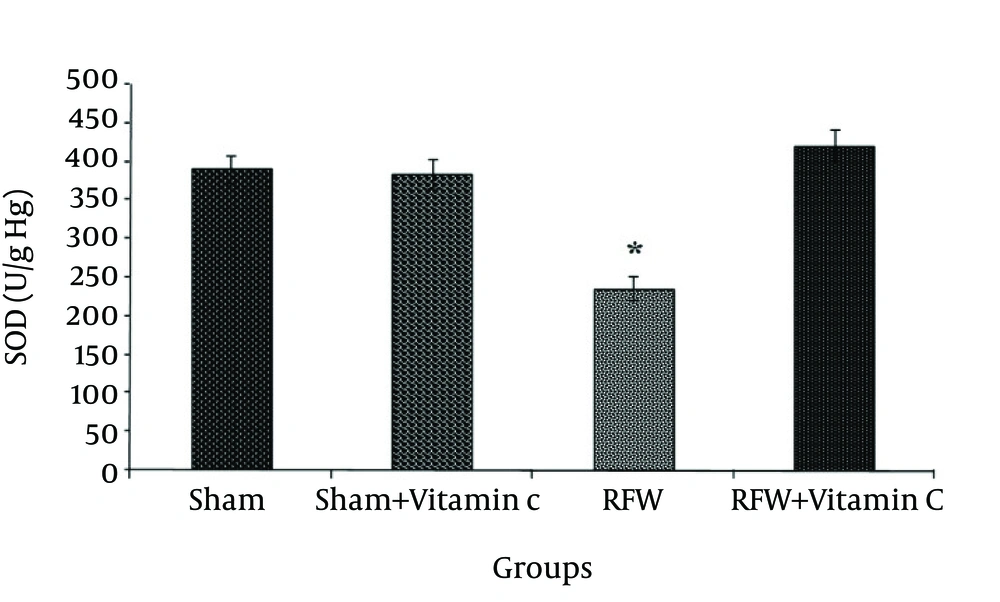
The mean values (±SEM) of GPx, SOD, CAT and MDA activity (as the biomarker for lipid peroxidation) in the rat erythrocytes are presented in figures 1-4. Exposure to EMF significantly decreased the activity of SOD in the RFW group compared to other groups, while administration of vitamin C could significantly increase the activity of this enzyme and bring it to normal level with no significant difference compared to the sham group (Fig. 1) (p<0.05).
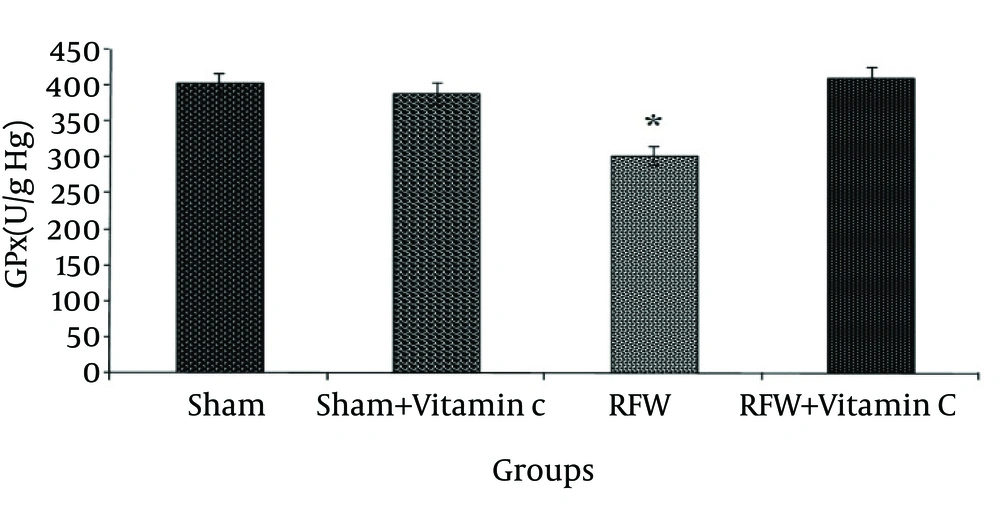
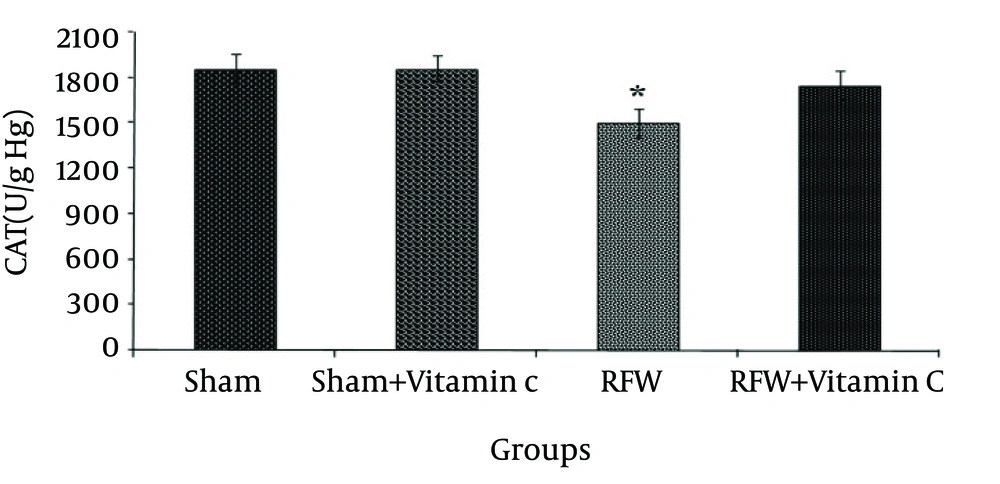
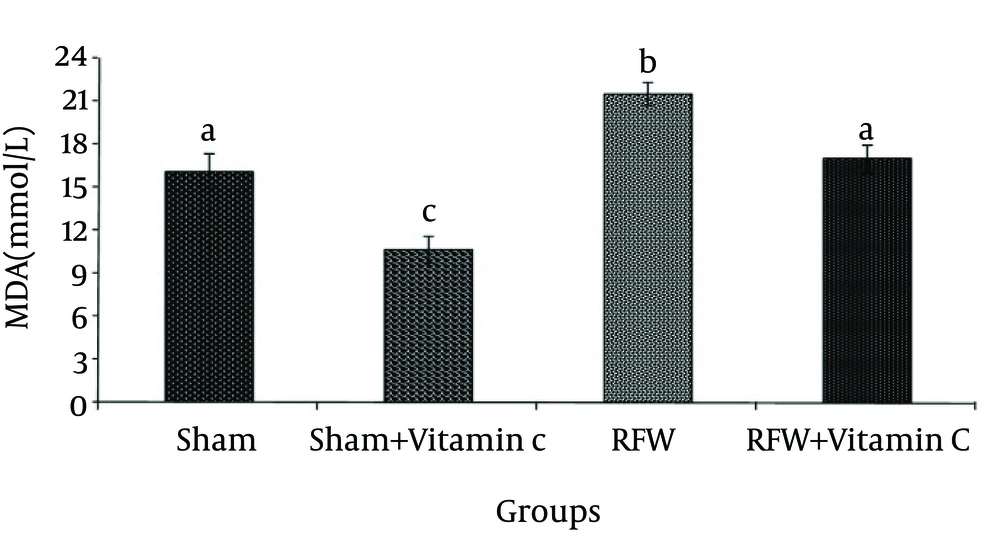
The sham- vitamin C group receiving vitamin C did not show significant changes in the activity of this enzyme compared to sham group. Exposure to EMF also significantly decreased the activity of GPx compare to sham group, and administrated vitamin C raised its activity to the normal level (Fig. 2) (p<0.001). CAT activity was significantly lower in the group exposed to RFW compared to the other groups (Figure 3) and pretreatment by vitamin C could have prevented this effect (p<0.05). Exposure of rats to RFW significantly increased lipid peroxidation products (as shown by MDA concentration) as compared to sham, while pretreatment of rats with vitamin C suppressed MDA concentration significantly (Fig. 4) (p<0.001).
Discussion
The results indicate that exposure to RFW in the test group decreased antioxidant enzymes activity and increased MDA compared with the control groups.
Indexes of oxidative stress in erythrocyte showed significant changes following exposure of animals to RFW. It is possible that non-thermal effects of electromagnetic waves from mobile phones increase the reactive oxygen species (ROS) in tissues and cells [1, 19, 20].
It is well known that ROS lead to oxidative damage in major cell macromolecules, such as lipids, proteins and nucleic acids, and is proposed to be the cause in tissue injury. ROS are scavenged by SOD, GSH-Px, and CAT. MDA is the byproduct of the major chain reactions leading to the oxidation of polyunsaturated fatty acids, and thus serves as a marker of oxidative stress-mediated lipid peroxidation.
The disruption of the oxidant/antioxidant balance in the erythrocyte and other tissues exposed to electromagnetic waves emitted from mobile phone has been shown in experimental studies [20-22]. Moustafa et al. [22] reported that exposure to radio wave (900 MHz) emitted from a mobile phone (for 1, 2 and 4 h/day for 30 days) significantly increase lipid peroxidation and decrease activity of SOD and GPx, while did not change catalase activity in human red blood cells. Agarwal et al. [23] reported that chronic exposure to electromagnetic radiation reduced the enzymes activity of SOD, GPx and catalase and increased lipid peroxidation. Also, Meral et al. [24] showed that exposure to electromagnetic fields emitted from mobile (12 h/day, for 30 days) increased production of free radicals and decreased the antioxidant enzymes activity and increased lipid peroxidation in the brain and blood. Our results showed that administration of vitamin C to exposed group could significantly reduce effects of oxidative stress.
Marković et al. [25] showed that vitamin C (500 mg/kg daily for 3 days) increased glutathione peroxidase activity and improved lipid peroxidation levels in red blood cells in rat. Vitamin C has a potent physiological role, very low standard reduction potential (282 mV), and is able to regenerate intracellular compounds such as glutathione, NADH and NADPH [11, 26]. Also, vitamin C removes hydrogen peroxidase and other free radicals, thereby adjusting the activity of glutathione peroxidase and catalase [27].
In addition, vitamin C is able to regenerate α-tocopherol from tocopherol radical species, thereby decreasing lipid peroxidation levels [11]. Vitamin C is capable to neutralize single oxygen and hydroperoxyl and peroxyl radicals and particularly superoxide anion [28] hence; improve of the activity of antioxidant enzymes. In conclusion, the results of this study suggest that RFW led to oxidative stress in erythrocyte and vitamin C improved antioxidant enzymes activity and decrease lipid peroxidation. There is a need for further study with different frequencies and exposure periods in order to discover the effects of radio frequency wave-induced oxidative stress in the cells.