1. Background
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide, characterized by an abnormal inflammatory response and chronic, irreversible, and persistent airway restriction symptoms (such as shortness of breath, coughing, and sputum production) (1, 2). Chronic obstructive pulmonary disease affects more than 329 million individuals globally, and it is estimated that by 2060, more than 5.4 million patients will die from COPD exacerbations and related diseases each year (3, 4).
Initial management of COPD patients includes lifestyle modifications, especially smoking cessation, vaccination, and regular exercise programs. However, due to excessive mucus secretion, tissue destruction, and disruption of normal repair and defense mechanisms, inhaled preparations with different pharmacologic effects, including inhaled corticosteroids (ICSs), Long-acting ß2-agonists (LABAs), and long-acting muscarinic receptor antagonists (LAMAs) are indicated for maintenance therapy (5-7). Additionally, treatment for patients with stage 3 and 4 COPD (severe and very-severe stages) includes long-term oxygen therapy and lung transplantation (8).
The clinical manifestations of COPD are associated with the three arms known as chronic bronchitis, emphysema, and asthma, which ultimately lead to airflow obstruction (9). Eosinophilic airway inflammation, a key feature of asthma, is a recognized inflammatory endotype in COPD (10-12). Chronic obstructive pulmonary disease with eosinophilic inflammation [also known as Asthma-COPD overlap (ASO)] is defined as sputum eosinophils ≥ 3% (13, 14). Assessment of eosinophilic airway inflammation using induced sputum samples is technically challenging and not always successful, decreasing the usefulness of the test in routine clinical practice. There is a reasonable correlation between blood eosinophil count and sputum count in COPD patients, so blood eosinophil count may be a more reasonable alternative (a differential count of 2% or higher in blood has a positive predictive value of 90% for a raised eosinophil count in induced sputum) (15, 16).
Inhaled corticosteroids are one of the main treatment options for reducing the risk of moderate to severe COPD exacerbations (17). Chronic obstructive pulmonary disease patients with predominantly eosinophilic airway inflammation (ACO) may benefit most from ICS administration. Nevertheless, the benefits and risks of ICS treatment remain controversial, especially given the increased risk of pneumonia (18, 19).
2. Objectives
Therefore, in this study, we determine the frequency of eosinophilia in COPD patients and compare the effects of ICSs in patients with and without eosinophilia referring to the lung clinics of Zahedan University of Medical Sciences.
3. Methods
This study was a prospective, longitudinal, single-blind clinical trial. The participants were COPD patients referred to the lung clinics at Ali Ebn Abitaleb Hospital, Zahedan, Iran. The diagnosis of COPD in these participants relied on patient history, physical examination, chest X-ray findings, and spirometry data. Inclusion criteria for COPD were as follows: (1) Airway signs and symptoms (such as productive cough, dyspnea, and wheezing); (2) confirmed chronic airway obstruction (defined as forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 70% and FEV1 < 80% of the predicted values by spirometry data, and FEV1 reversibility after inhalation of 200 μg salbutamol of < 12% of prebronchodilator FEV1); (3) over 40 years of age; (4) patients who had been stable for 4 weeks prior to the initial visit and received optimal medical therapy. Exclusion criteria for participants included the coincidence of cardiovascular disease, eosinophilia-related disease (e.g., parasites, collagen-vascular disease, hematologic, and oncological disease), and COPD exacerbation within 4 weeks prior to the first visit.
To determine the sample size, we utilized the formula for assessing the superiority of the mean 6-minute walking test (6MWT) after the study compared to the baseline.
Using the results of a pilot study containing four eligible patients (ɑ = 0.05, ß = 0.25, σ = 14.5, ɛ = 9.5, δ = 5) where σ, ɛ, and δ are the standard deviation (SD) of the differences, mean differences, and the superiority margin for 6MWT, respectively, 58 patients were computed and selected to compare the effectiveness of ICSs. Eligible patients were selected after ethical approval of the plan by the Research Ethics Committees of Zahedan University of Medical Sciences (IR.ZAUMS.REC.1397.224) and the Iranian Registry of Clinical Trials (IRCT20181126041758N1). First, peripheral blood smears (PBS) were obtained from all patients, and based on the percentage of blood eosinophils, they were divided into 2 groups (the threshold was 2% eosinophils). A 6MWT was then performed on all patients, wherein the patient walked a distance of 30 meters in 6 minutes. During this test, the person was allowed to stop if they felt tired or experienced symptoms (like dyspnea, chest pain, etc.) until they were able to resume walking. Thereafter, two groups of patients received standard treatment with the Symbicort spray for 3 months (manufactured by AstraZeneca, France and distributed by Cobel Darou, Iran). They were followed for the period, and the 6MWT was performed again at the end of the 3-month period. After data collection, the information was entered into SPSS software version 26. Comparisons of treatment effects for parametric data were performed by independent t-tests; otherwise, non-parametric equivalence tests such as Mann-Whitney were used.
4. Results
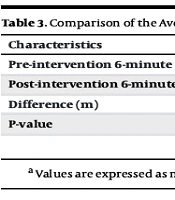
Among 58 COPD patients, 49 patients (84.5%) had negative eosinophilia, and the rest (9 patients, 15.5%) had positive eosinophilia. The mean age of the patients was 58.81 ± 14.4 years. Out of 58 patients, 34 (58.6%) were male, and 24 (41.4%) were female. There were no significant differences in the mean age (P = 0.66) and gender (P = 0.72) between patients with and without eosinophilia (Table 1). The average FEV1 and FEV1/FVC of COPD patients with positive eosinophilia were 52.78 ± 20.66% and 62.14 ± 20.9, respectively. In addition, the FEV1 and FEV1/FVC of COPD patients with negative eosinophilia were 62.06 ± 18.58 and 74.89 ± 19.43, respectively (Table 2). The mean 6MWT test before and after intervention in the eosinophilia-positive group was 164.02 ± 108.97 meters and 192.11 ± 101.02 meters, respectively. Therefore, there was a significant difference in patients’ activity levels before and after taking ICSs (P = 0.017, Table 3). Furthermore, in the eosinophilia-negative group, there was a significant difference in patient activity levels based on the 6MWT test before and after taking ICSs (147.9 ± 54.62 meters and 169.22 ± 60.47 meters, respectively, P < 0.001). However, there was no significant difference in activity levels between the two groups at baseline and also after the intervention (P = 0.68 and P = 0.36, respectively).
| Variables | Negative Eosinophilia | Positive Eosinophilia | P-Value |
|---|---|---|---|
| Age | 58.4 ± 14.9 | 60.7 ± 11.8 | 0.66 |
| Sex | 0.72 | ||
| Male | 28 (57.1) | 6 (66.7) | |
| Female | 21 (42.9) | 3 (33.3) |
a Values are expressed as mean ± SD or No. (%).
| Characteristics | Eosinophilia Positive | Eosinophilia Negative | P-Value |
|---|---|---|---|
| Forced expiratory volume in 1 | 52.78 ± 20.66 | 62.06 ± 18.58 | 0.38 |
| Forced expiratory volume in 1/forced vital capacity | 62.14 ± 20.9 | 74.89 ± 19.43 | 0.33 |
a Values are expressed as mean ± SD.
| Characteristics | Eosinophilia Positive | Eosinophilia Negative | P-Value |
|---|---|---|---|
| Pre-intervention 6-minute walking test (m) | 164.02 ± 108.97 (80.2 - 247.7) | 147.9 ± 54.62 (132.2 - 163.59) | 0.68 |
| Post-intervention 6-minute walking test (m) | 192.11 ± 101.02 (114.4 - 269.7) | 169.22 ± 60.47 (151.85 - 186.59) | 0.36 |
| Difference (m) | 28.08 ± 27.91 (6.63 - 49.5) | 21.32 ± 19.95 (15.58 - 27.25) | - |
| P-value | 0.017 | < 0.001 | - |
a Values are expressed as mean ± SD (95% CI).
5. Discussion
Chronic obstructive pulmonary disease is a progressive disease often associated with irreversible respiratory complications. It is one of the most prevalent diseases globally, imposing significant morbidity on societies, economics, and health systems worldwide. Currently, the primary focus in COPD management is to identify markers that aid in developing the best treatment plan for each patient, determining the response to the chosen plan, and predicting the prognosis of the disease (1). Although several studies have explored the relationship between eosinophilia and clinical outcomes in COPD patients, the benefits and risks of ICS treatment remain controversial. This study aims to investigate the efficacy of ICS treatment in COPD patients based on blood eosinophilia. The main findings of this study are as follows: (1) Treatment with ICS resulted in a significant difference in the 6MWT test between the eosinophilia-positive and eosinophilia-negative groups; (2) treatment with inhaled β2-agonists plus corticosteroids did not affect the response to this treatment in subjects' groups with and without eosinophilia compared to the pre-treatment conditions.
Our results indicate that there are no significant differences in patient activity levels based on the 6MWT test before and after taking ICSs between the two groups (the eosinophilia-positive and the eosinophilia-negative group). These findings align with those of Yousuf et al., who showed that there is no significant relationship between eosinophilia, health, and COPD complications in patients (20). However, a cohort study by Jabarkhil et al. (21) demonstrated that patients with eosinophilic COPD had no increased risk of disease progression compared to COPD patients without eosinophilia. In contrast to these results, Kitaguchi et al. found in a study that patients with COPD and asthmatic components, who had higher eosinophilia and sputum eosinophilic count than non-asthmatic COPD patients, showed a better response to ICSs treatment. It was concluded that initiating treatment earlier in these patients could be associated with a better response and greater reversibility (22). Another study demonstrated that in COPD patients with eosinophilia, treatment with inhaled β2-agonists plus corticosteroids is associated with a decrease in the frequency of acute attacks of the disease (23). Siddiqui et al. showed that peripheral blood eosinophilia in COPD patients is a predictor of the desired response to long-acting inhaled β2-agonists plus corticosteroids, which may be attributed to its inflammatory profile and better response to corticosteroid therapy (24).
These differences may arise because COPD and asthma can overlap, a situation known as Overlap Syndrome. Patients with this syndrome are presumed to exhibit different clinical characteristics and better responses to ICS treatments. The chronic airway inflammation in COPD differs significantly from asthma, which is typically characterized by inflammation and hyperresponsiveness throughout the airway without involvement of the lung parenchyma (25). Asthma patients who smoke may develop pathological features similar to COPD. Similarly, some COPD patients may manifest asthma-like symptoms, including a complex inflammatory pattern and increased eosinophilia. It has been reported that in COPD, an elevated eosinophil count in sputum is associated with an improvement in FEV1 following treatment with ICSs (26, 27).
While eosinophil count may serve as a suitable and accessible biomarker for COPD treatment and predicting response to ICSs (28), potential barriers such as comorbidities, the patient’s current dosing regimen, and the lack of proven correlations between peripheral blood eosinophils and tissue eosinophils limit their use in routine medical therapy.
5.1. Conclusions
In this study, it was observed that despite significant differences in the activity levels of patients in the 6MW test after treatment with inhaled β2-agonists plus corticosteroids in both patient groups with and without eosinophilia compared to pre-treatment conditions, the response to this treatment in subjects with peripheral blood eosinophilia showed no significant difference compared to patients without eosinophilia. Additionally, there were no significant differences in age and gender in this study.
5.2. Recommendations
Considering the smaller sample size of patients in the eosinophil-positive group compared to the eosinophil-negative group, we recommend prospective multicenter randomized clinical trials with both smaller and larger sample sizes. Additionally, due to the limited feasibility of patient follow-up, we suggest conducting a similar study with full-time access to the patients to evaluate their response to treatment.