1. Background
Non-alcoholic fatty liver disease (NAFLD), one of the most common causes of chronic liver disease, is characterized by fat accumulation in the liver. It ranges in severity from simple hepatic steatosis or fatty liver to non-alcoholic steatohepatitis (NASH) (1). This disease has a high prevalence; it affects 2.8 - 40% of people worldwide (2) and 33.9% of the Iranian population (3). It is associated with central obesity, advanced age, hypertension, dyslipidemia, metabolic syndrome, and inflammation, which can lead to cardiovascular diseases (CVD) and, eventually, increased all-cause mortality (4, 5).
In recent decades, diet has been considered an important factor in the development of NAFLD (6). Many epidemiological studies have examined associations between the intake of separate nutrients or foods and NAFLD risk (7). However, in reality, people do not consume nutrients separately; rather, they consume their meals as a combination of foods and nutrients (8). Based on current trends in clinical nutrition and nutritional epidemiology, analysis of dietary patterns is considered a more accepted approach to determining the relationship between diet and chronic disease because of its ability to examine the effects of the total diet (9).
Recent studies on dietary patterns and NAFLD have shown that adherence to certain dietary patterns, such as the dietary approaches to stop hypertension (DASH) diet (10) and the Mediterranean diet (MED), may reduce the risk of NAFLD (11, 12). Evidence also suggests that the Healthy Eating Index-2015 (HEI-2015) has a significant inverse correlation with insulin resistance, liver fat, and metabolic parameters associated with NAFLD (13).
2. Objectives
Given the widespread prevalence of NAFLD and its serious complications, and due to the paucity of published information on the relationship between dietary patterns and NAFLD risk in the Iranian population, we conducted this study to investigate possible associations between NAFLD risk and adherence to the MED, DASH, and HEI-2015 diets.
3. Methods
3.1. Participants
The present case-control study was conducted between November 2018 and May 2019 on 122 NAFLD patients and 121 non-NAFLD patients referred to a gastroenterology clinic in Ahvaz, Iran. The participants were aged 19 to 70 years. The exclusion criteria were all types of disabilities, chronic diseases such as cancer, diabetes, alcoholic liver disease, viral hepatitis, hepatotoxicity due to drug abuse, and alcohol consumption of more than 10 g/day in women and 20 g/day in men (14). All the participants signed the informed consent form before entering the study. In addition, the study protocol was approved by the Ethics Committee of the Shoushtar School of Medical Sciences based on the 1975 Declaration of Helsinki (ethics code: IR.SHOUSHTAR.REC.1401.015).
The diagnostic criteria for NAFLD were elevated levels of alanine aminotransferase (ALT) (normal range: 29 to 33 IU/L in men and 19 to 25 IU/L in women), aspartate aminotransferase (AST) (normal range: 10 to 40 IU/L in men and 9 to 32 IU/L in women), and confirmation of fatty liver by ultrasonography (15). The control group and the NAFLD group were compared for sex, body mass index (BMI), and age (at five-year intervals). A body mass index of 18.5 - 24.9, 25 - 29.9, and more than 30 kg/m2 was classified as normal weight, overweight, and obese, respectively (16). Ultrasonography was performed for all the participants. No hepatic steatosis was observed in the control group.
3.2. Measurements
The participants completed a demographic questionnaire. Body weight was measured to the nearest 0.5 kg, without shoes and at least without clothing. Height was also measured with a tape measure to the nearest 0.1 cm. The BMI was calculated by dividing weight (kg) by the square of height (m). Waist circumference was measured with a tape measure to the nearest 0.1 cm above the iliac crest. Blood pressure was measured after a rest period of 10 minutes using a standardized blood pressure monitor in the sitting position. The use of antihypertensive medication, a systolic pressure > 140 mmHg, and a diastolic pressure > 90 mmHg were defined as hypertension. A validated questionnaire was used to measure physical activity, defined as metabolic equivalent hour/day (MET-h/day) (17).
3.3. Dietary Assessment
A semiquantitative and valid 147-item food frequency questionnaire (FFQ) was used to assess the participants' dietary habits (18). Food frequency was reported daily, monthly, and annually in the past year. Then, the reported food data (in household measures) were converted into daily intake (in grams).
3.4. Dietary Patterns
Trichopoulou et al.'s method was applied to calculate the MED diet score (19). The MED diet score was determined based on 8 food groups, with a maximum score of 8. One score was assigned to the participants if they consumed equal to or more than the median daily intake of vegetables, fruits, whole grains, nuts, fish, legumes, and monounsaturated fatty acids (MUFA) to saturated fatty acids (SFA) in grams. On the other hand, 1 score was given to the participants for below-median intake of dairy products and all types of meat (poultry, red meat, and processed meat). Overall, the participants' MED score was good, with a high intake of vegetables, fruits, fish, legumes, whole grains, and nuts and a low intake of dairy products and meat.
Fung et al.'s method was adopted to calculate the DASH score (20). This method was also based on 8 food groups. One score was assigned to the participants with the highest quartile for consumption of vegetables, fruits, whole grains, nuts, legumes, and low-fat dairy products. On the other hand, 1 score was assigned to the participants with the lowest quartile for daily intake of processed and red meat, sodium, and sweetened beverages. Overall, the participants had higher DASH scores when they consumed a greater amount of vegetables, fruits, legumes, low-fat dairy products, whole grains, and nuts and had a lower intake of processed and red meats, sodium, and sweetened beverages.
The HEI-2015 score is based on 13 components, including 9 components with adequate consumption of fruits, vegetables, seeds, dairy products, whole grains, total protein, seafood, vegetable protein, and fatty acids (polyunsaturated fatty acids (PUFA) + monounsaturated fatty acid (MUFA) / saturated fatty acids (SFAs), and 4 components with moderate consumption of refined grains, added sugars, and SFAs (21). For the 9 components mentioned, a maximum (highest intake) and minimum (lowest intake) score of 5 and 0 were given, respectively, with the exception of whole grains, dairy products, and fatty acids, which were given a maximum score of 10 for the highest intake. For the 4 components mentioned above, the highest score (lowest intake) was 10, and the lowest score (highest intake) was 0.
Finally, each individual's score for dietary patterns was obtained by summing the scores of the food groups. Higher and lower scores showed higher and lower adherence to dietary patterns, respectively. Then, according to the scores, the individuals were categorized into pentiles of adherence for HEI-2015, DASH, and MED dietary patterns.
3.5. Statistical Analysis
All the analyses were run using IBM SPSS v. 22 (IBM Corp., Armonk, NY, USA). The participants' characteristics were reported using a chi-square test for categorical variables and an independent samples t-test for continuous variables. Multivariable logistic regression was used to investigate the association between the risk of NAFLD and adherence to HEI-2015, DASH, and MED dietary patterns. Furthermore, adjustment of covariates was performed for age, sex, BMI, total energy intake, physical activity, educational status, and smoking. P-values > 0.05 were considered statistically significant.
4. Results
Table 1 shows the general characteristics of the participants. One participant (in the NAFLD group) was excluded because of an overestimation of calorie intake (more than 3 standard deviations from the mean). Thus, the data of 243 participants were analyzed.
The NAFLD patients had significantly higher waist circumference (WC) and energy intake compared with the control group (P < 0.001). Furthermore, there were significantly more smokers and fewer educated subjects in the NAFLD group compared with the control group (P < 0.05).
| Variables | Non-alcoholic Fatty Liver Disease (NAFLD) (N = 121) | Control (N = 122) | P-Value b |
|---|---|---|---|
| Age (y) | 42.95 ± 11.46 | 42.51 ± 11.52 | 0.70 c |
| Sex | 0.93 d | ||
| Female | 64 (52.9) | 64 (52.5) | |
| Male | 57 (47.1) | 58 (47.5) | |
| Height (cm) | 165.53 ± 10.16 | 165.97 ± 9.19 | 0.54 c |
| weight (kg) | 81.78 ± 13.12 | 80.76 ± 13.28 | 0.67 c |
| BMI (kg/m2) | 30.53 ± 5.04 | 29.32 ± 4.49 | 0.08 c |
| Physical activity level (metabolic equivalent/hour/day) | 34.11 ± 5.87 | 35.94 ± 7.88 | 0.14 c |
| Educational level | 0.002 d | ||
| Illiterate | 14 (11.6) | 2 (1.6) | |
| Elementary school | 36 (29.8) | 30 (24.6) | |
| High school diploma | 34 (28.1) | 31 (25.4) | |
| University | 37 (30.6) | 59 (48.4) | |
| Waist circumference (cm) | 102.86 ± 10.78 | 98.08 ± 10.55 | < 0.001 c |
| Total energy intake (kcal/day) | 4122.76 ± 1624.85 | 3178.60 ± 936.18 | < 0.001 c |
| Systolic blood pressure (mmHg) | 124.09 ± 12.29 | 121.02 ± 14.45 | 0.32 c |
| Diastolic blood pressure (mmHg) | 81.35 ± 6.96 | 80.14 ± 6.44 | 0.28 c |
| Marital status | 0. 72 d | ||
| Married | 105 (86.8) | 102 (83.6) | |
| Single | 16 (13.2) | 20 (16.4) | |
| Smoking | 0.04 d | ||
| Yes | 12 (9.9) | 4 (3.3) | |
| No | 109 (90.1) | 118 (96.7) |
a Values are presented as No. (%) or mean ± SD.
b P-value < 0.05 was considered significant.
c P-value based on the independent samples t-test.
d P-value based on the chi-square test.
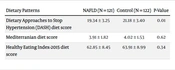
| Dietary Patterns | NAFLD (N = 121) | Control (N = 122) | P-Value |
|---|---|---|---|
| Dietary Approaches to Stop Hypertension (DASH) diet score | 19.34 ± 3.25 | 21.18 ± 3.40 | 0.01 |
| Mediterranian diet score | 3.91 ± 1.82 | 4.02 ± 1.53 | 0.62 |
| Healthy Eating Index-2015 diet score | 62.85 ± 8.45 | 63.91 ± 8.99 | 0.34 |
a Values are presented as mean ± SD.
b P-value based on independent samples t-test
c P-value < 0.05 was considered significant.
Table 2 shows a comparison of the mean scores of dietary patterns in NAFLD and control groups. The control group had a significantly higher DASH diet score compared to the NAFLD group (P = 0.01).
Table 3 presents the association of the quintiles of adherence to MED, DASH, and HEI-2015 dietary patterns with the NAFLD risk. Three models were considered. Model 1 as a crude model; Model 2 was adjusted for sex, age, BMI, and total calorie intake; and Model 3 was adjusted for sex, age, BMI, total calorie intake, WC, smoking, educational status, and physical activity.
| Variables | Q1 | Q3 | Q5 | P-Trend b |
|---|---|---|---|---|
| MED | ||||
| Model 1 | 1 (ref.) | 1.36 (0.53 - 3.46) | 0.73 (0.3 - 1.79) | 0.42 |
| Model 2 | 1 (ref.) | 0.59 (0.20 - 1.75) | 0.47 (0.17 - 1.32) | 0.15 |
| Model 3 | 1 (ref.) | 0.63 (0.20 - 1.97) | 0.51 (0.17 - 1.52) | 0.23 |
| DASH diet | ||||
| Model 1 | 1 (ref.) | 0.39 (0.14 - 1.02) | 0.37 (0.14 - 0.92) | 0.03 |
| Model 2 | 1 (ref.) | 0.33 (0.11 - 0.98) | 0.34 (0.12 - 0.94) | 0.03 |
| Model 3 | 1 (ref.) | 0.30 (0.09 - 0.95) | 0.33 (0.11 - 0.96) | 0.04 |
| HEI-2015 | ||||
| Model 1 | 1 (ref.) | 0.58 (0.23 - 1.46) | 1.13 (0.47 - 2.74) | 0.77 |
| Model 2 | 1 (ref.) | 0.56 (0.20 - 1.53) | 1.13 (0.43 - 2.98) | 0.80 |
| Model 3 | 1 (ref.) | 0.79 (0.27 - 2.32) | 1.45 (0.53 - 3.96) | 0.46 |
Abbreviations: BMI, body mass index; WC, waist circumference; MED diet, Mediterranean diet; DASH, Dietary Approach to Stop Hypertension; HEI, Healthy Eating Index.
a Logistic regression, model 1: Crude; model 2: Adjusted for age, sex, BMI, and energy intake; model 3: Adjusted for age, sex, BMI, energy intake, WC, smoking, physical activity, and educational status.
b P-value < 0.05 was considered significant.
In regards to DASH diet adherence, the risk of NAFLD was lower in Models 1, 2, and 3 odds ratios (OR) (OR = 0.37, 95% CI = 0.14 - 0.92; OR = 0.34, 95% CI = 0.12 - 0.94; OR = 0.33, 95% CI = 0.11 - 0.96, respectively) in the highest quintile compared to the lowest quintile. However, no significant correlation was observed between NAFLD risk and adherence to MED and HEI-2015 dietary patterns.
5. Discussion
The findings showed that the highest adherence to the DASH dietary pattern was associated with decreased odds of NAFLD risk. However, no significant relationship was observed between MED and HEI-2015 dietary patterns and the risk of NAFLD. To the best of our knowledge, this study is the first to evaluate the relationship between the risk of NAFLD and the DASH, MED, and HEI-2015 scores in Ahvaz, Iran.
A few studies have investigated the relationship between healthy diet scores and NAFLD risk. A cross-sectional study conducted by Xiao et al. showed that the highest adherence to the DASH diet scores is associated with a significant decrease in NAFLD risk (10). This finding is consistent with the results of our study. A randomized clinical trial (RCT) demonstrated that the DASH dietary pattern, when followed for 8 weeks, exhibited beneficial effects on hepatic enzymes in NAFLD patients compared to a standard diet (22). A multiethnic cohort study highlighted a negative correlation between DASH scores and the risk of NAFLD (23), which is similar to our results. In contrast to our findings, Hekmatdoost et al., in their case-control study, observed no significant association between adherence to the DASH diet and the risk of NAFLD after adjustment for BMI and other confounding variables (24). This discrepancy might be due to differences in the patients' age (25).
As the DASH dietary pattern is characterized by high consumption of fruits, vegetables, unsaturated oils, whole grains, and low-fat dairy, and a low intake of red meat and sweets, it can decrease body weight and steatosis and regulate liver enzymes (26). According to evidence, the DASH diet improves risk factors of NAFLD, such as type 2 diabetes mellitus (T2DM), obesity, metabolic syndrome (MetS), and dyslipidemia. It may also prove to be a more effective dietary approach for weight management compared to other weight loss diets. The DASH diet has the potential to enhance serum levels of triglycerides, ALT, AST, insulin, and inflammatory factors (27). Mechanisms of the effects of the DASH diet on the decrease in NAFLD risk include 1) a low intake of added sugars, particularly fructose, by activating hepatic lipogenesis; 2) high fiber intake, along with energy restriction for weight loss and improvement of gut microbiota (28); 3) low intake of sodium and saturated and trans-fatty acids; 4) high amount of antioxidants and nutrients as a factor in NAFLD prevention; and 5) low-fat dairy products, especially fermented dairies which have probiotics (24).
Recent studies have yielded conflicting findings regarding the relationship between the MED dietary pattern and the risk of NAFLD. A prospective study has demonstrated a significant relationship between adherence to the MED dietary pattern and a reduction in cardiovascular risk factors and Fatty Liver Index (FLI) in NAFLD patients in Greece (28). However, a case-control study conducted in Iran displayed no significant association between adherence to the MED dietary pattern and cardiovascular risk factors (29). On the other hand, a recent systematic review and meta-analysis of clinical trials showed the significant effect of the MED diet on the reduction of FLI in NAFLD patients (30).
Although the MED dietary pattern is characterized by a high intake of whole grains, fruits, vegetables, and unsaturated fatty acids, our study did not find a significant correlation between adherence to the MED dietary pattern and the risk of NAFLD. The difference in the outcomes could potentially be attributed to the geographical area of investigation. As there exists a disparity in the dietary patterns of the Mediterranean regions and other regions such as Iran, the findings of the studies could be controversial. For instance, in Iran, cereals are predominantly prepared using refined grains, whereas in Mediterranean countries, cereals are primarily made using whole grains (31, 32). There also exists a disparity in the culinary techniques employed for cooking fish in Mediterranean and non-Mediterranean nations. It appears that individuals residing in Mediterranean regions have a higher consumption of omega-3 fatty acids compared to other regions. This can be attributed to the prevalent use of olive oil in the preparation of fish dishes, which is more common in Mediterranean countries as opposed to regions such as Iran. In Iran, sunflower or corn oil is more commonly used than olive oil for preparing fish, primarily due to economic considerations. Olive oil may offer superior protection for the nutritional value of fish when compared to sunflower or corn oil due to its higher content of unsaturated fatty acids (29, 32, 33).
Limited research has been conducted on the correlation between HEI-2015 scores and the risk of NAFLD. Hashemi Kani et al. conducted a case-control study in which they found no significant correlation between quartiles of adherence to HEI-2015 scores and the risk of NAFLD (34), which is consistent with the outcome of our study. The multiethnic cohort study yielded significant findings regarding the relationship between adherence to pentiles of HEI-2015 scores and the risk of NAFLD (23). This difference in findings may be attributed to variations in sample size and population demographics.
The HEI-2015 diet is characterized by increased consumption of fruits, vegetables, seeds, dairy products, whole grains, total proteins, seafood, herbal proteins, and fatty acids (PUFA + MUFA / SFAs), as well as moderate intake of grains, added sugar, and SFAs (21). Therefore, enough intake of herbal protein, fiber, and antioxidants such as vitamin E, vitamin C, and other natural antioxidants could reduce the risk of NAFLD by detoxification and prevention of steatosis in the liver (34).
This study represents an initial investigation into the association between the risk of NAFLD and adherence to DASH, MED, and HEI-2015 dietary patterns in Ahvaz, Iran. The study employed a validated semi-quantitative FFQ based on the dietary habits of the Iranian population. Potential confounding variables were accounted for and adjusted for in the analysis. However, the present study also had certain limitations. This research employed a case-control design. Therefore, one cannot establish a causal relationship between dietary scores and the risk of NAFLD. Furthermore, the data were collected using an FFQ as a self-reporting questionnaire, which may introduce certain biases, such as recall bias, that are likely to occur.
5.1. Conclusions
This study demonstrated that the Ahvaz population with the highest adherence to the DASH diet exhibits the lowest risk of NAFLD. No significant correlation was found between MED and HEI-2015 dietary scores and the risk of NAFLD. It is imperative to conduct additional studies with larger samples and across diverse communities to validate the findings of this study.