Introduction
Over the years, doxorubicin (DOX), as an anti-tumor antibiotics and anticancer [1-6] widely used as a agent in various cancers treatment [7, 8]. Unfortunately, the clinical use of these drugs is limited, because of severe toxic effects of the drug on the body's tissues, including heart, liver, kidneys and nervous system. The mechanisms of DOX-mediated cytotoxicity in cancer cells and normal tissues are different. Cytotoxicity may play a central role in DOX-induced cardiotoxicity or hepatotoxicity [7, 9-12]. In this regard, considerable effort has been in medical and sports field [1-6, 13, 14] to develop strategies to prevent DOX-induced toxicity, especially in heart tissue. Babaei et al. [4] reported the protective effect of morphine on DOX-induced cardiotoxicity. However, few studies have been conducted on the toxicity of DOX in liver tissue. The insulin-like growth factor (IGF) system is an essential regulator of growth and development [15]. In normal conditions, the levels of the components reach a balance, so that the IGF axis plays a critical role in cellular proliferation as well as cell survival [16, 17]. The bioavailability of IGFs is modulated by high-affinity binding proteins known as insulin-like growth factor binding proteins (IGFBPs) (from 1 to 7), that are mainly synthesized in liver. Most of the circulation IGF-1 is carried by IGFBP-3 [15]. IGF system plays a critical role in cell proliferation. Including IGF-1, IGF-2 and family of proteins that binds to IGF (IGFBP), which is specialized bind to IGF [16]. Thus, there is growing evidence to suggest that IGF system is used as a panel of tumor markers for histological diagnosis of various diseases associated with cancer. In this regard, it was reported that insulin-like growth factor (IGF-1) and protein-3 binds to IGF (IGFBP-3) protein-1 binds to IGF (IGFBP-1) are associated with an increased risk of breast cancer in premenopausal women [18]. However, it is not known whether exercise could affect IGF-1 levels and its binding proteins.
Although, there are few longitudinal studies regard the effect of training on IGF-1 proteins, it is not clear whether regular aerobic training could affect the IGF system in the liver tissue. Furthermore, it is not clear that DOX-induced hepatotoxicity by 2 doses of 10 and 20 mg/kg affect of the hepatic IGF system and whether pretreatment with regular aerobic training can affect changes in these parameters? While, many recent studies have focused on the treatment effect of endurance exercise on induce DOX cardiotoxicity [19, 20], the present study was carried out to investigate effect of prior (pretreatment) regular aerobic training against doxorubicin-induced hepatotoxicity with various dosages (10 and 20 mg/kg) on insulin-like growth factor (IGF) signaling system (IGF-I, IGFBP-3 and IGF/IGFBP).
Materials and Methods
Experimental Design: The empirically protocols of this experimental study approved by department of physiology, university of Mazandaran and were performed according to guiding procedures in the care and use of animals, prepared by the Council of the American Physiological Society. The experiments were carried out with 48 Wistar male rats (8-week-old, initially weighing 257±28 g), which were obtained from the laboratory of animal bearing and multiplying at the Pasture institute of Iran. Rats were housed in standard cages of polycarbonate (20×15×15 cm), made at the Pasture institute of Iran, in a large air-conditioned room with a controlled temperature of 22±2ºC, light-dark cycles of 12:12 h and humidity of 50±5%. The pollutant standard index (PSI) was in the acceptable range as determined by the Iranian meteorological organization. Rats were fed with a standard rat chow provided by Pars institute for animals and poultry with a daily regimen of 10 g per 100 g body weight for each rat. Water was available ad libitum.
Familiarization and aerobic training protocols: Animals were habituated to treadmill running for one week (once a day for 10 min/session at 10 m/min, 0% grade). Because rats are more active in darkness, the front portion of the treadmill lines was covered with a dark thick paper to darken this area. At the rear of the lines, an electric grid provided a stimulus for running. An electric stimulus (30 V, 0.5 A) was manually turned on for less than 2 s when the animals stayed on the electric grid for longer than 10 s. Rats quickly learned to stay on the belt and avoid shock, except for one rat, which would not stay on the moving belt, and thus was quickly removed from familiarization process. Following this familiarization period, they were randomly assigned into control and trained groups. Exercise training protocol was performed on treadmill with 0 slopes between 25 to 54 min/session and 15 to 20 m/min, 5 days/week for 3 weeks (Table 1). We replicated the aforesaid exercise training protocol that was previously reported by Dabidi-Roshan et al. [21].
Classification of rats: At the end of the exercise training protocol, rats from the control and trained groups were again randomly separated into subgroups; the DOX (10, 20 mg/kg) and placebo treatment. Thus, the control rats were distributed into control+placebo (group 1, N=8), control+DOX (group 2, N=8) and control+DOX (group 3, N=8) groups and rats in the trained group into trained+placebo (group 4, N=8), trained+DOX (group 5, N=8) and trained+DOX (group 6, N=8) groups.
Doxorubicin treatment: Doxorubicin hydrochloride (EBEWE Pharma Ges.m.b.H.Nfg.KG) was dissolved in saline and administered by i.p. injection at 2 dosages of 10 mg/kg [22] and 20 mg/kg [23], and control animals received saline with comparable volume. Both treatments were carried 24 h after the last exercise bout and animals were sacrificed 24 h after DOX and placebo injections.
Liver tissue collection and preparation: Rats in all groups were anesthetized with ketamine and xylazine following 24 h of DOX injection and 12 h fasting. The abdominal cavity was opened to expose the liver tissue. Then liver tissue were rapidly excised, rinsed, carefully dried, weighed and it was placed into Petri dishes containing cold isolation medium (0.1 M/L K2HPO4, 0.15 M/L NaCl, pH=7.4) to remove the blood and were frozen immediately in liquid nitrogen and stored at -80ºC. Liver tissue was squashed in liquid nitrogen, homogenized in a lysis buffer (5 mL/g of tissue) with a protease inhibitor cocktail for mammalian cell and tissue extracts (Sigma-Aldrich, St. Louis, U.S.A) 100 uL/1 mL, and 10 mM tris base (Sigma-Aldrich, St. Louis, USA), pH=7.4 and centrifuged at 1500 g at 4ºC for 15 min. Hepatic supernatant was diluted 1:30. Plasma was diluted 1:10 homogenized in doubly distilled water. Homogenates were centrifuged (2 min at 2,000 g, 4ºC) to eliminate cellular debris, and the resulting supernatant was stored at liquid nitrogen (-80ºC) for later determination of insulin-like growth factor (IGF) system include insulin-like growth factor (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3).
Quantitative detection of the IGF system: Markers of the IGF system (IGF-I and IGFBP-3) were measured using the following ELISA kits according to the manufacturer's instructions: IGF-1 ELISA kit (DRG International, USA) and IGFBP-3 Quantikine ELISA Kit (R&D Systems, USA). Absorbance was read at 450°nm for the three kits in a microplate reader. In summary, 100 µL of standard, blank, or sample added per well. The liquid removed of each well and 100 µL of biotin-antibody working solution added to each well. Then, aspirated each well and washed and repeated these step for three times.In addition, 100 µL of HRP-avid in working solution added to each well and the aspiration and washing repeated five times as step 4. Moreover, 90 µL of TMB substrate added to each well. Also, 50 µL of stop solution added to each well when the first 4 wells containing the highest concentration of standards develop obvious blue color. Finally, the optical density of each well was determined within 30 min; a microplate reader set to 450 nm was used.
Statistical Analysis: All data have been expressed as mean±SD. Statistical analysis was performed using a commercial software package (SPSS-20 for Windows). Data of the IGF system were normally distributed after log-transformation. A one-way analysis of variance (Statistics software, Stat Soft, Inc., Tulsa, OK) was used to detect statistical differences between groups. A post-hoc test (Tukey test) was performed to determine differences in the various biomarkers between groups. Differences were considered statistically significant at p-value<0.05.
Result
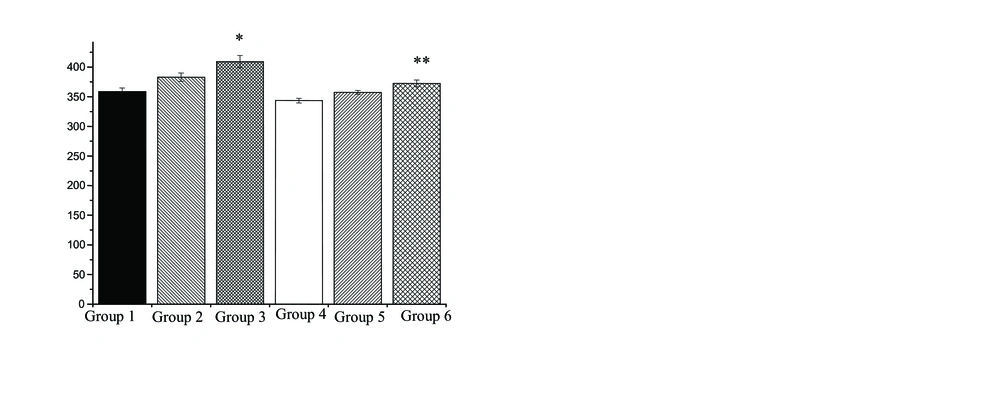
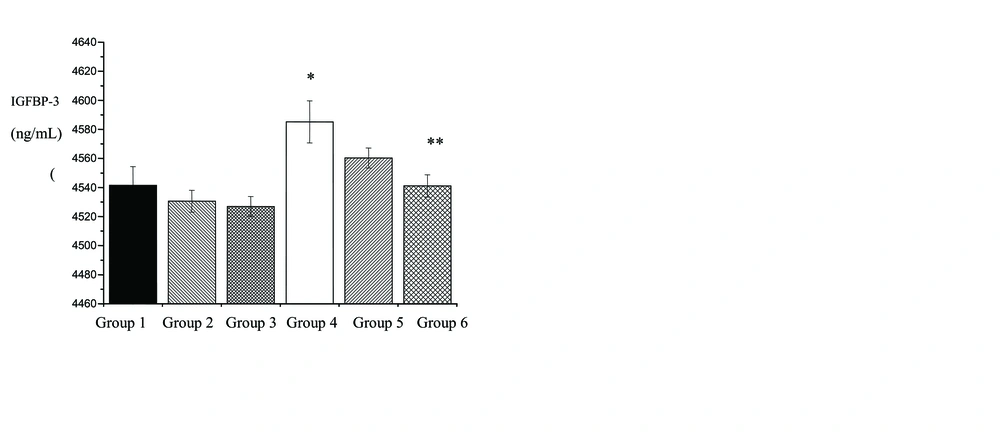
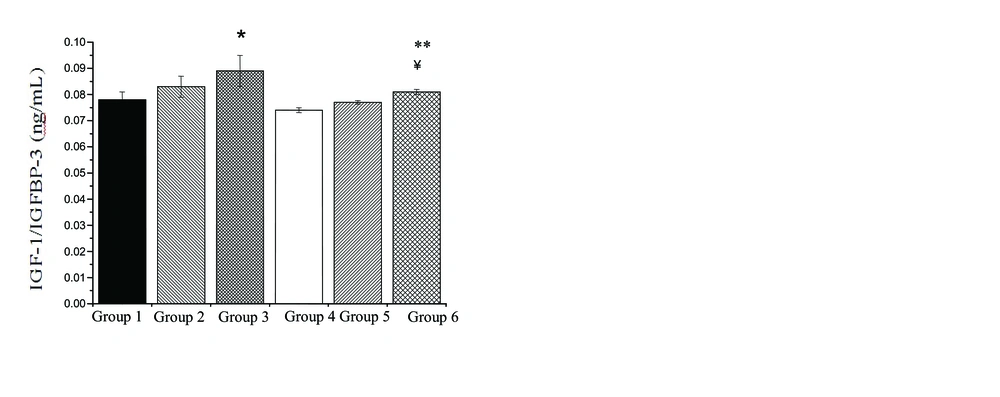
No difference existed in the age and weight values between rats in the various groups. Changes in IGF-1, IGFBP-3 and IGF-1/IGFBP-3 levels following doxorubicin treatment (DOX 10 mg/kg, DOX 20 mg/kg) in the various groups showed in figures. Rats in the control group, showed an insignificant increase in IGF-1 and IGF-1/IGFBP-3 (6.8% and 6.41%, respectively), an insignificant decrease in IGFBP-3 (0.04%), following DOX 10 mg/kg administration. However, administration of DOX 20 mg/kg in control group caused a significant increase in IGF-1 (p=0.05) and IGF-1/IGFBP-3 (p=0.01) (14% and 14.1%, respectively), an insignificant decrease in IGFBP-3 (0.3%) in comparison to rats in the group 1. Also, there was no significant difference between DOX 10 mg/kg and DOX 20 mg/kg treatments in IGF-1, IGFBP-3 and IGF-1/IGFBP-3 levels.
Figures 1, 2 and 3 show changes in IGF-1, IGFBP-3 and IGF-1/IGFBP-3 levels following protective role of 3 weeks of aerobic training in the rats exposed to DOX-induced hepatotoxicity. Three weeks of the regular aerobic training led to an insignificant decrease in IGF-1 (p=0.02) and IGF-1/IGFBP-3 (p=0.01) (26% and 5.1%, respectively) and a significant increase in IGFBP-3 (96%), as compared to group 1 (p=0.03).
Changes in the IGF-1, IGFBP-3 and IGF-1/IGFBP-3 levels, shows in figure 1, 2 and 3. After 3 weeks of aerobic training and DOX treatment with 10 mg/kg, an insignificant decrease in IGF-1 and IGF-1/IGFBP-3 (6.7% and 7.2%, respectively), and an insignificant increase in IGFBP-3 (45%), were detected in comparison to c+DOX 10 group. In addition, protective role of regular aerobic training and DOX treatment with 20 mg/kg resulted in a significant decrease in IGF-1 (p=0.02) and IGF-1/IGFBP-3 (p=0.01) levels (8.9% and 8.9%, respectively), an insignificant increase in IGFBP-3 (0.29%), as compared to c+DOX 20 group.
Furthermore, after 3 weeks of aerobic training and doxorubicin treatment (10 mg/kg), an insignificant increase in IGF-1 and IGF-1/IGFBP-3 levels (4.01% and 4.05%, respectively), and an insignificant decrease in IGFBP-3 (0.5%), were detected in comparison to group 4. After 3 weeks of aerobic training and doxorubicin treatment (20 mg/kg), an insignificant increase in IGF-1 (8.4%), a significant increase in IGF-1/IGFBP-3 (9.4%) (p=0.002) and a significant decrease in IGFBP-3 (9%), were detected in comparison to group 4 (p=0.05). However, after 3 weeks of aerobic training and DOX 10 mg/kg and DOX 20 mg/kg treatments there was no significant difference between in IGF-1, IGFBP-3 and IGF-1/IGFBP-3 levels.
| Weeks Training days | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 Speed (m/min) | 15 | 15 | 15 | 15 | 15 |
| Time (min) | 25 | 26 | 27 | 28 | 29 |
| 2 Speed (m/min) | 16 | 16 | 16 | 16 | 16 |
| Time (min) | 30 | 31 | 32 | 33 | 34 |
| 3 Speed (m/min) | 17 | 17 | 17 | 17 | 17 |
| Time (min) | 35 | 36 | 37 | 38 | 39 |
Discussion
The present study was designed to determine protective role of regular aerobic training on the IGF system (IGF-1, IGFBP-3 and IGF-1/IGFBP-3) in the rats exposed to DOX-induced hepatotoxicity with the various dosages (10 and 20 mg/kg). The primary novel finding in present study was that, administration of DOX 10 mg/kg and DOX 20 mg/kg led to imbalance in the IGF signaling system in rats. This result indicates potent role of DOX administration in induce oxidative stress. Doxorubicin is an anthracycline antibiotic that is considered as 1 of the most effective antitumor agent. The clinical use of DOX is limited by toxicity to normal tissues, such as the heart and liver [7, 24]. The mechanisms of DOX-mediated cytotoxicity in cancer cells and normal tissues are different [7]. On the other word, DOX-induced cardiotoxicity or hepatotoxicity mainly occurs by generating oxygen free radicals, which is inhibited by free radical scavengers [7]. Oxidative stress plays a major role in DOX-induced cardiomyopathy and hepatic damage. Numerous methods have been used to minimize the cardiotoxic effects of the chemotherapeutic agent doxorubicin, and most have had limited success. Chronic endurance exercise has been shown to protect against DOX hepatotoxicity, but little is known the effects of acute exercise on DOX-induced hepatic dysfunction [25]. There is growing evidence to suggest that the IGF system are used as a panel of tumor markers for histological diagnosis of various diseases associated with cancer [24].
Most tumor cell types possess IGF1 receptors and conditions in the tumor micro environment, such as hypoxia, can lead to enhanced responsiveness to IGF1. Therefore, inhibiting IGF1 action has been proposed as a specific mechanism for potentiating the effects of existing anticancer therapies or directly inhibiting tumor cell growth. IGFBP3 is the most abundant IGFBP in blood and has the highest affinity for IGF1 and IGF2, therefore it accounts for 75-80% of the total carrying capacity. Because of its role as a generalized systemic growth factor and because many tumor cell types possess IGF1 receptors, targeting IGF1 has been extensively studied in the context of cancer research. Early experimental findings showed that breast cancer cells expressing IGF1 receptors were responsive to this growth factor [26]. In a study IGF and IGFBP was introduced as an indicator for the detection of tumor growth. The researchers said these factors play a pivotal role in tumor formation. Epidemiological data suggest that the risk of cancer is associated with high levels of IGF-1. Lack of regulation of IGF system can cause problems in some tissues, IGF correct pattern and its binding proteins, thus may be useful as a new tumor marker for cancer detection and evaluation [16]. We also confirmed that acute administration of DOX increased IGF-1 levels and IGF-1/IGFBP-3 ratio and decreased IGFBP-3 values in liver tissue of male rats. Our results are consistent with several published reports on the levels of IGF signaling system in the rats exposed to DOX-induced hepatotoxicity. Also, our data demonstrated that acute administration of DOX increased serum indices of liver function, including ALT (Alanine transaminase) and ALP (Alkaline phosphatase). This increase in ALT and ALP is attributed to the hepatocellular damage and decreased liver functions. The present results indicate that there is a potent relationship between IGF system and DOX-induced hepatotoxicity.
The American Cancer Society recently published guidelines recommending that all cancer patients be encouraged to exercise during chemotherapy. These recommendations are primarily based on the promising preliminary evidence of the effects of exercise on maintaining or enhancing quality of life. As such, studies investigating the potential interaction between exercise and chemotherapy efficacy are essential to the interpretation and acceptance of exercise as a modifier of quality of life. As expected, groups that received doxorubicin had significantly prolonged tumor growth delay than groups who did not receive cytotoxic therapy. Exercise is becoming readily accepted as a beneficial adjunct therapy to maintain or enhance quality of life in breast cancer patients undergoing adjuvant chemotherapy. An essential precursor to these studies is to investigate whether exercise modulates the antitumor efficacy of chemotherapeutic agents [27].
No studies have been previously to determine protective role of regular aerobic training on the IGF system (IGF-1, IGFBP-3 and IGF-1/IGFBP-3) in the rats exposed to DOX-induced hepatotoxicity with the various dosages (10 and 20 mg/kg). Our study confirmed that that pretreatment of regular aerobic training prevented down-regulation of IGFBP-3 and up-regulation of IGF-1 in liver tissue. In the present study, a significant decrease in the IGF-1 level and IGF/IGFBP-3 ratio in rats treated with t+DOX treated groups, suggests the protective and pretreatment effect of regular aerobic training against DOX-induced hepatotoxicity.
While, previous researchers have reported physical activity as a non-pharmacological strategy in various cancers, we are the first to investigate the pretreatment effect of regular aerobic training before the various dosages (10 and 20 mg/kg) of DOX on markers of related to hepatotoxicity in liver tissue. Our study demonstrated, although, 20 mg/kg of DOX can lead to down-regulation of the IGF signaling system, regular aerobic training increase IGFBP-3, decrease IGF-1 and IGF-1/IGFBP-3 which is induced by DOX administration. In contrast with these results, Andrea et al. examine the effect of 16 weeks of aerobic training on IGF axis proteins in active young women. They reported the baseline values in subjects IGFBP-3 had little significant increase. Researchers concluded that 16 weeks of aerobic training in young women has no effect on IGF proteins [28]. After 10 minutes exercise IGFBP-3 was only significantly increased after high-intensity training, whereas IGF-1 was not affected by any of the interventions [29].
High serum concentrations of insulin-like growth factor-1 (IGF-1) are associated with chemo-resistance in colorectal cancer, whereas IGF binding protein-3 (IGFBP-3) seems to exert a pro-apoptotic effect [30].
The resting IGF-1/IGFBP-3 ratio was significantly higher in athletes. These results indicate that elevation of baseline serum IGF-1/IGFBP-3 ratio after exercise might suggest that free fractions of these hormones may act as a potent stimulant of muscle hypertrophy in trained endurance athletes. Graded exercise tests had a significant effect on serum IGF-1 concentration at maximal intensity of exercise.
Furthermore, at maximal exercise, higher values of serum IGF-1, IGFBP-3, and IGF-1/IGFBP-3 ratio were found in athletes compared to control group [30]. Sprod et al. [31] found an inverse relationship between changes in IGF-1 and changes in overall health related quality of life, physical role limitations, and social functioning. Changes in IGFBP-1 and IGFBP-3, binding proteins for IGF-1, were correlated with changes in physical role limitations and physical functioning, respectively.
Early epidemiological evidence does not support an inverse association between chronic exercise and circulating levels of IGF1 and IGFBP3, particularly in older women. A recent review also suggested that long term exercise may actually increase circulating IGF1. Therefore, the evidence is weak that chronic exercise lowers IGF-1 levels [32].
In conclusion, the present findings demonstrate that the hepatotoxicity induced by DOX 20 mg/kg, may be related to imbalance of IGF system in liver tissue. Moreover, protective role of 3 weeks aerobic training effectively improve the toxic effects of DOX in liver, it was associated with up-regulation of IGFBP-3 and down-regulation of IGF-1. Overall, our study suggests that short-term regular aerobic training before administration of DOX may be considered as a potentially useful strategy to limit hepatotoxicity before DOX therapy and improve IGF system in liver tissue. The present findings demonstrate that the hepatotoxicity induced by DOX 20 mg/kg, may be related to imbalance of IGF system in liver tissue.
Moreover, protective role of 3 weeks aerobic training effectively improve the toxic effects of DOX in liver, so that it was associated with up-regulation of IGFBP-3 and down-regulation of IGF-1. Overall, our study suggests that short-term regular aerobic training before administration of DOX may be considered as a potentially useful strategy to limit hepatotoxicity before DOX therapy and improve IGF system in liver tissue.