Introduction
Epilepsy is a neurological disorder in which the repeated unpredictable seizures are symptomatic and affects about 0.5-0.7% of the population worldwide [1]. Our understanding of the epileptogenic process originates mainly from animal studies that model the process of epileptogenesis [2]. Among those, kindling as an epilepsy model develops gradually the susceptibility of the brain neuronal networks to epileptogenesis [3]. The kindling-induced plastic changes up-regulate the excitatory system, whereas reduce the inhibitory system function. This results in a net excitability of the brain. For this purpose, different kinds of electrical or chemical stimuli are used to cause the kindling paradigm, and the chemical substance, pentylenetetrazol (PTZ) induces convulsions in animal by repeated injection [4-6].
The stress experiences could have a profound effect in shaping an organism’s physiological response [4, 5]. Also, stress induces different changes in the behavior achieved through the modulation of neuronal function involved in different aspects of hormonal and neural responses [6]. Furthermore, exposure to stress induces cognitive and neuroendocrine responses [5, 7] as well as development of hypothalamic pituitary adrenal (HPA) and other neuroendocrine axes. In response to acute and chronic stressors, the autonomic nervous system and the HPA axis start to react in order to maintain homeostasis. The activation of the HPA axis in turn affects the adrenal steroids and the production of neurosteroids. These changes have been shown to alter seizure susceptibility in humans as well as animal models [8].
Exercise affects the overall health and the brain function; from increased levels of growth factors, the stimulation of neurogenesis, the increase in resistance to the brain insult and improvement of learning and mental performance, to the alteration of gene expression in plasticity, metabolic, anti-aging and immune functions [9]. A meta-analysis showed that the older adults with cognitive impairments participating rehabilitation exercise, experienced an enhanced cognitive function suggesting a positive effect of exercise [10]. On the other hand, exercise is a kind of physical stress which might mimic the devastating effect of stress on brain and body. Whether exercise is helpful, harmful, or simply has no impact on seizure frequency has been debated for years. For instance, exercise shows a positive effect on seizure frequency and severity [11] and raises seizure threshold as a protective effect in people with epilepsy. Conversely, a few patients complain that the stress during sports exacerbates their seizures [11, 12]. Therefore a few physicians warn them against vigorous exercise because it might precipitate seizures [13]. Even some stress models involving activity sessions might have exercise effect on epileptogenesis. Thus, the aim of this study is to investigate the effect of different acute and chronic stresses as well as exercise on the kindling development of PTZ treated animals.
Materials and Methods
In this experimental study, total of 82 male Wistar rats (Iran Pasteur Institute) weighing 180 g at the beginning of experiments were used. All experiments were done in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 23-80, revised 1996) and conformed to the research ethical standards for the care and use of animals in Damghan Branch Islamic Azad University. Rats were kept 5 per cage and all were under normal 12 h light, 12 h darkness (lights on at 07:00 am) with food and water ad labium.
Rats were randomly sorted into 7 experimental groups: (1) PTZ kindling of intact animals (N=14); (2) Forced swimming stress (FSS) in 25°C water for 30 min (N=14); (3) FSS in 20ºC water for 30 min (N=14); (4) FSS in 32°C water for 30 min (N=12); (5) FSS in 20ºC water for 30 min up to 5 days (N=13); (6) Swimming in 25°C water, 60 min of a day and 5 days per week for 8 weeks (N=7); (7) Swimming in 32°C water, 60 min of a day and 5 days per week for 8 weeks (N=8). The convulsive scores of all groups were compared to PTZ kindling group as a control, while different temperatures were compared to convulsive scores in 25°C.
All rats were subjected to either swimming exercise or stress in a plastic container (40×45×45 cm) under continuous supervision with the water set to different temperatures. Changing water temperature from 25ºC (control temperature), is considered a stress effect which enhances or reverses the background effect in stress and exercise models, respectively. Stress groups animals tolerated 30 min swimming stress with different water temperatures of 20°C [14], 25°C [15, 16] and 32°C [16] as in the acute model. The chronic stress was induced by repetition of acute swimming stress only for 5 days [17] in 20°C water. Severe stress was produced by only decreasing water temperature of acute stress to 20°C cold temperature. The reason for lowering the temperature in the chronic stress model is to ensure the chronic nature of stress and also to remove the probable exercise effects created by the repeating of the acute model. The exercise was induced by swimming in 25 and 32ºC water, 60 min/day, 5 times per week during 8 weeks (modified from) [18-20]. The duration of swimming was initially 10 min and was gradually increased by 10 min/day until it reached a maximal of 60 min that was maintained during all the training period [21].
Male Wistar rats were selected to start the kindling procedure with 180 g of weight (young age), one day after the termination of the stress or exercise model. PTZ (Sigma; cat, P6500), 40 mg/kg, dissolved in 1 mL distilled water and each rat received 13 intraperitoneal (i.p.) injections every 48 h. Rats were considered kindled after 3 consecutive 4-5 convulsions, which were scored (modified from Racine et al. [22]) as follows; 0, no response; 1, ear and facial twitching; 2, convulsive waves through the body; 3, myoclonic jerks; 4, tonic-clonic (TC) convulsions, rearing; 5, generalized tonic-clonic seizures, turnover into side position and loss of postural control [23]. The time average of 13 convulsive stages in kindled and non-kindled animals was used to compare the excitability of rat's brain [24]. The first 5 injections kindling stages (the start of network changes) were averaged as an index of kindling threshold and last 5 injections (establishment of kindling process) averaged to represent the kindling rate.
The time averages of first and last 5 convulsive stages in stressed and exercised animals were used as a criterion to compare the kindling threshold and the kindling rate, respectively. The kindling period with the averaged values were compared using Kruskal Wallis non-parametric test which was then followed by Dunn`s post-hoc when appropriate. The data will be presented without unit, for they are in fact seizure stage as ordinal data. All of statistical tests were performed by statistical SPSS-16 software. Data presented as mean±SEM. Minimum significant level was set at p<0.05.
Results
The swimming stress and exercise are 2 kinds of physical activity which affect the convulsive behavior. Comparing different stress and exercise effects using Kruskal Wallis non-parametric analysis showed a significant difference between kindling groups in terms of the kindling threshold (p=0.05), and the kindling rate (p=0.05).
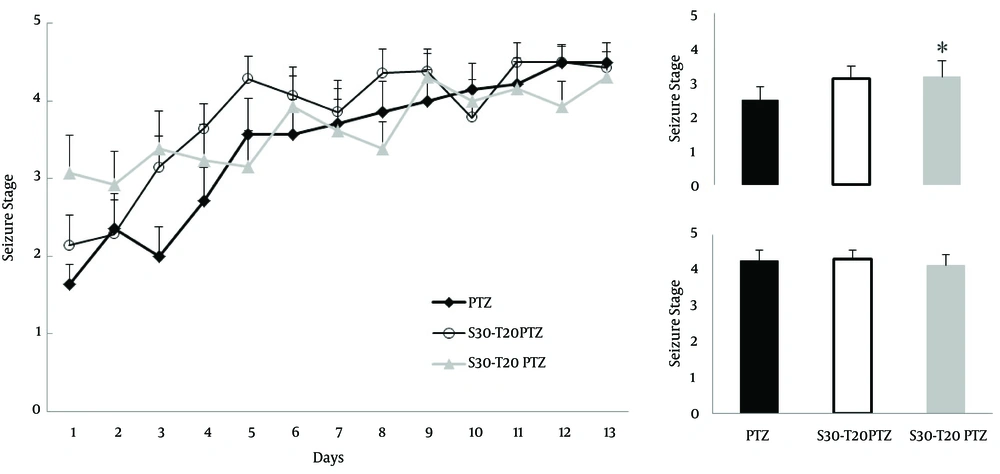
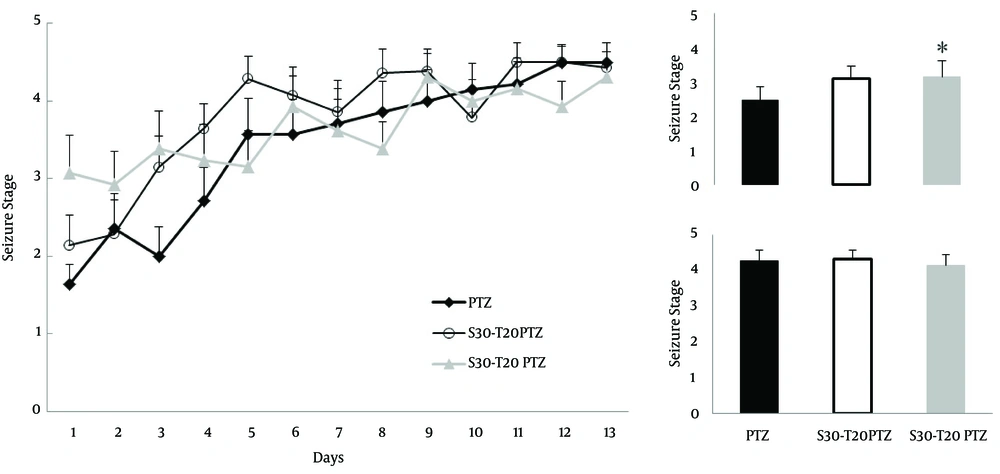
The convulsive behavior of male rats, forced to swim in 25 and 32ºC water for 30 min as acute stress, was compared using Dunn`s method which showed that the swimming stress in 25°C water (4.2±0.2 vs. 3.6±0.1) decreased the kindling rate (p=0.03, N=14, Fig. 1), but had no significant effect on the kindling threshold (2.4±0.2 vs. 2.25±0.2). In contrast, swimming stress in 32ºC water (2.4±0.2 vs. 3.31±0.3) revealed a significant decrease in the kindling threshold (p=0.035, N=12, Figure 1) without concomitant change in the kindling rate (4.2±0.2 vs. 4.01±0.2).
The adverse effects of stress are often more pronounced in the chronic application. So, Dunn`s method comparison revealed that chronic stress (2.4±0.2 vs. 3.1±0.3) only reduced kindling threshold, significantly (p=0.031, N=13, Fig. 2), while kindling rate did not change (4.2±0.2 vs. 4.1±0.2). Severe stress did not change kindling threshold (2.4±0.2 vs. 3.1±0.3) or kindling rate (4.2±0.2 vs. 4.3±0.2). Thus, the results showed that repeated application of an acute stress paradigm affected kindling more vigorously than enhancing the process.
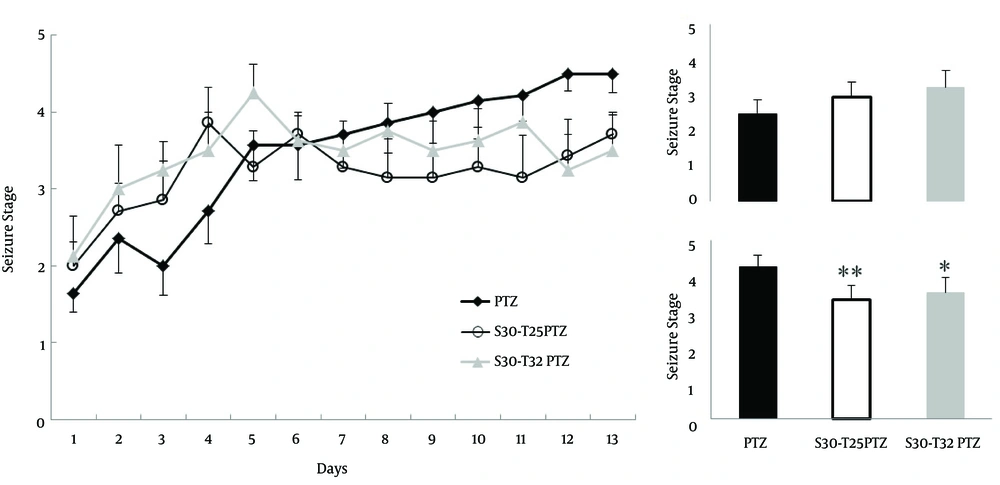
Although it is accepted that exercise triggers convulsions, many experiments showed the beneficial effect of exercise in seizure relief. Comparing exercise effect on convulsive behavior in 2 temperatures using Dunn`s method revealed that 8 weeks exercise in 25°C water (4.2±0.2 vs. 3.3±0.3) reduced kindling rate (p=0.007, N=7, Fig. 3), considerably. A significant decrease in kindling rate (p=0.02, N=8, Fig. 3) was demonstrated due to 8 weeks exercise in 32°C water (4.2±0.2 vs. 3.5±0.09). In contrast, the kindling threshold did not change due to exercising in 25ºC (2.4±0.2 vs. 2.9±0.3) water, nor did it change in 32°C (2.4±0.2 vs. 3.2±0.3) temperatures. Therefore, our data demonstrated that exercise reduced the kindling rate but not the kindling threshold.
Acute stress reduced the resistance against PTZ induced convulsions. Thirty minutes swimming in 32°C water revealed higher stage convulsions in first 5 injections (inset; upper chart). Swimming stress for 30 min in 25°C water reduced convulsion intensity in last 5 injections (inset; lower chart). Inset; ordinal average of first 5 injections (Upper; Kindling threshold) and last 5 injections (Lower; Kindling rate), PTZ, Pentylenetetrazol; S, Stress; T, Temperature; * p<0.05
Chronic stress decreased kindling threshold of PTZ induced kindling. Five day repeat of 30 min swimming in 20°C water revealed higher stage convulsions in first 5 injections (inset; upper chart). Severing swimming stress, for 30 min in 20°C water, did not change convulsion intensity in last 5 injections (inset; lower chart). Inset; ordinal average of first 5 injections (Upper; Kindling threshold) and last 5 injections (Lower; Kindling rate), PTZ, Pentylenetetrazol; S, Stress; T, Temperature; D, Day; * p<0.05
Regular exercise decelerated PTZ induced kindling. Swimming regular 8 week exercise in 25°C water reduced kindling progression in last 5 injections (inset; lower chart). Also, swimming exercise in 32°C water decreased convulsion intensity in last 5 injections (inset; lower chart). Inset; ordinal average of first 5 injections (Upper; Kindling threshold) and last 5 injections (Lower; Kindling rate), PTZ, Pentylenetetrazol; Ex, Exercise; T, Temperature; * p<0.05; ** p<0.01
Discussion
This study investigated the differential effect of stress and exercise on PTZ induced kindling development measured by kindling threshold and kindling rate. Acute and chronic stress reduced the kindling threshold compared to control, untreated animals, while regular medium swimming exercise decreased the kindling rate and hence kindled number.
Swim stress is shown to exacerbate convulsion intensity following acute and chronic stresses in animals [8], and also increasing mortality and morbidity in patients with epilepsy due to emotional and psychological stresses [25]. We demonstrated a decrease in the kindling threshold of acute 32°C water and also chronic five days swim stresses of 20°C water. A similar pro-convulsive result was demonstrated by Edwards et al. in which adult male offspring of prenatally stressed dams were kindled faster than non-stressed animals [4]. The responsible mechanism for this pro-convulsive effect might be the enhancing effect of different stress models on oxidative stress [26] governed by PTZ convulsions [27]. In contrast to the pro-convulsive effect, there are reports of attenuating convulsive intensity in rats [28] and mice [14]. This is in agreement only with our acute 25°C water swim stress data which demonstrated a decrease of kindling rate of PTZ treated rats. The controversy with other results might be due to milder effect of swim stress in room temperature rather than cold situation [29].
Physical activity is considered one of the most important and accessible factors to prevent and protect brain functions at low cost [30]. Our data demonstrated that regular moderate swimming exercise decreased kindling rate in both 25 and 32°C water. Although exercise is a physical activity in nature, it is shown that aerobic exercise improves cognitive function [31] and anxiety related behavior [19] and prevents age dependent cognitive decline [32]. Exercise also increases the volume of hippocampus [32] as well as neurotrophins, neurotransmitters and neuropeptides in different brain areas [33], hence improving memory and cognitive capacity [32, 34]. Such cognitive improvements are mainly through the increases of serum brain derived neurotrophic factors (BDNF) [9, 31, 35], which remains elevated even after exercise ceases [36]. This BDNF production might be the responsible mechanism for exercise induced kindling rate deceleration in our study. On the other hand, seizure increases oxidants and concomitant oxidative damage, while swimming exercise has been shown to increase antioxidant defenses and reduce basal production of oxidants such as Na, K-ATPase inhibition induced by PTZ [27].
Although, the lack of significant change of kindling threshold indicates the physical straining nature of exercise in the first days [37], there was a tendency to decrease kindling threshold in both 25 and 32°C water temperature swimming exercise in this time period. Thus, exercise as physical stress will exacerbate seizures while continuing exercise will reduce convulsive intensity and kindling rate.
It is concluded that swim stress facilitated kindling development in acute and chronic models, while exercise without a prominent effect on kindling threshold, reduced kindling rate at the final stage of kindling process.