1. Background
Sepsis is a significant cause of morbidity and mortality in all age groups (1, 2). It comes from a complex host response to an infecting microorganism, is exaggerated by the patient's endogenous factors, and mainly presents with decreased perfusion of blood to organs and multiple organ failure (3, 4). Any delay in treating this life-threatening condition is unacceptable and can lead to death (5). The prompt initiation of effective antimicrobial treatment is a crucial factor in the therapy of these patients (6). Therefore, antibiotics are usually advised empirically before the availability of blood culture results (7, 8). One of the most worldwide health concerns in recent years is the increasing frequency of antimicrobial resistance among microbial pathogens. Several classes of antimicrobial agents have become less effective, often due to extensive and inappropriate antimicrobial agent usage (9). The antibacterial sensitivity of microorganisms is mainly influenced by geographical location and changes with time. Accordingly, it is essential to continually review and update the microbiology and resistance patterns of relevant agents of the infections (7, 8). Despite the importance of rapid and highly effective antibiotic therapy in septicemic patients, few well-designed studies have been conducted on the susceptibility profile of causative agents of community-acquired sepsis worldwide. Previous studies have reported the antimicrobial sensitivity of positive blood culture isolates in hospitals. There was no plan to confirm sepsis (10-16), exclude contaminant results, or differentiate community-acquired from health care-associated infections (12-14, 16, 17). Certain studies have been conducted with a limited number of participants (17, 18), while others have exclusively focused on pediatric populations (12, 19).
2. Objectives
The present study aimed to report the etiology and antibiotic susceptibility of community-acquired septicemia in Isfahan, Iran.
3. Methods
3.1. Study Design
The present study was part of a large cross-sectional surveillance named IAS-1 (Isfahan Antimicrobial Resistance Surveillance-1), which was done in 3 major referral hospitals in Isfahan, Iran (Alzahra, Dr Shariati, and Dr Gharazi hospitals) (12).
All patients who were admitted to the hospitals and had bloodstream infections were assessed for enrollment in the study. Finally, those with community-acquired sepsis were included in the study. Bloodstream infection was defined as the growth of a true pathogen on one occasion or the growth of normal flora of the skin on 2 occasions with the same antibiotic susceptibility in blood culture. True sepsis pathogens were considered as Streptococcus pneumonia, Haemophilus influenza type b, Neisseria meningitides, Staphylococcus aureus, enteric gram-negative bacteria (Escherichia coli, Klebsiella spp., Proteus spp, Enterobacter spp, and Salmonella spp.), Pseudomonas aeruginosa, Acinetobacter spp, group A Streptococcus, and Enterococcus spp. Normal flora of the skin included coagulase-negative Staphylococci (CONS), Bacillus spp, Micrococcus spp, and Diphtheroid spp. Sepsis was defined as the presence of systemic inflammatory response syndrome (SIRS) in addition to the growth of a responsible pathogen in blood culture bottles. SIRS was defined by observation of at least 2 of the following criteria: A temperature of more than 38°C, elevated WBC (ie, ≥12 000/µL in adults and ≥15 000/µL in children), unexplained tachypnea, and unexplained tachycardia or hypotension. The existence of at least one of the first 2 criteria was required for the diagnosis of SIRS.
Patients with health care-associated sepsis, which was defined as positive blood culture after 48 h of admission with a new symptom such as fever, tachypnea, hypotension, etc, were excluded from the study (12).
3.2. Organism Identification and Antibiotic Susceptibility Testing
Blood samples for culture were prepared by trained nurses with aseptic techniques and inoculated in BACTEC or conventional blood culture bottles. Organism identification was done using conventional methods, and antimicrobial susceptibility pattern determination was performed and interpreted according to Clinical Laboratory and Standard Institute (CLSI-2016-2017) recommendations (20). Available dehydrated antibiotic discs from MAST, Merseyside, UK, were used in the study. Antibiotic strips from Liofilchem, Italy, were used for the determination of minimum inhibitory concentrations (MIC) of colistin and vancomycin. The kits and methods were similar in all enrolled laboratories.
3.3. Statistical Analysis
Data on sex and age group (< or = 20 years, > 20 years) in addition to microbiology and antibacterial sensitivity were obtained from WHONET dataset software version 5.6 in each microbiology laboratory and examined with SPSS version 18.0. Chi-square and Fisher’s exact tests were used to analyze categorical variables. A P-value below 0.05 was considered statistically significant.
3.4. Ethical Consideration
This study was approved by the Ethics Committee of Isfahan University of Medical Sciences (code: IR.MUI.MED.REC.1399.233).
4. Results
From a total number of 7238 cultivated blood samples, 6055 (83.6%) had no growth, 15 (0.2%) were positive for yeast, and 541(7.5%) were from patients suspected of health care-associated infection. Of the 627 community-acquired bloodstream infections, 480 (76.6%) patients had sepsis. Of the patients with community-acquired sepsis, 59.8% were male, and 11.0% were under the age of 20.
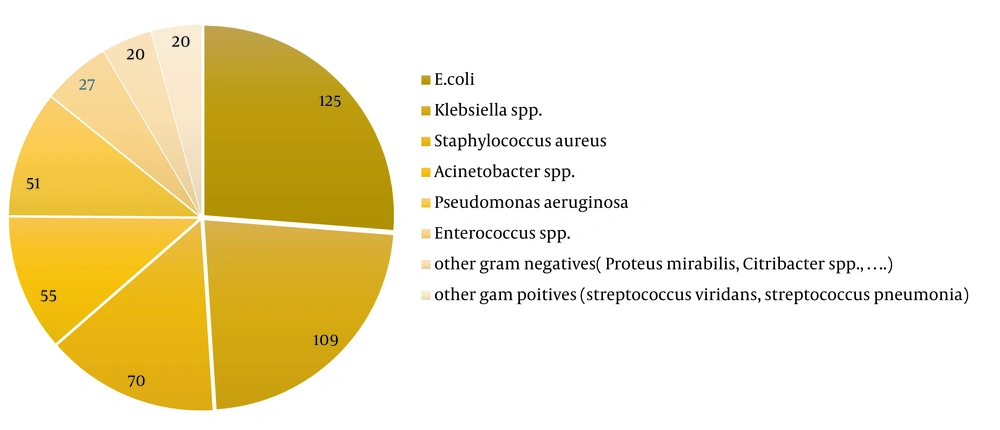
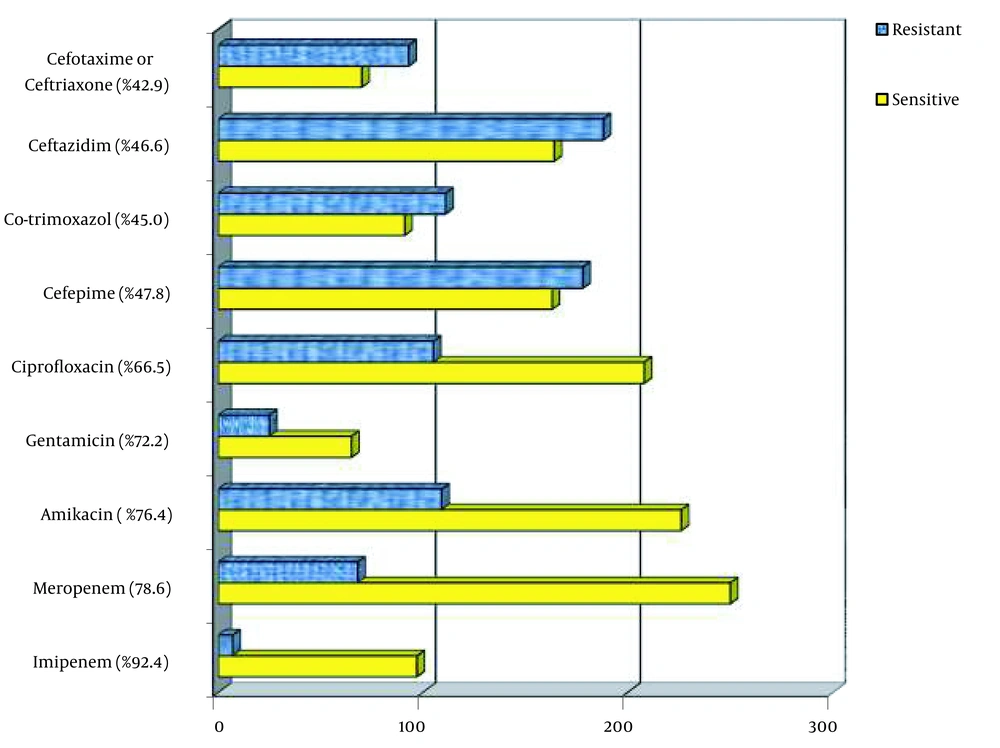
Gram-negative bacteria represented 361 isolates; among them, E. coli (26.3%) was the leading pathogen, followed by Klebsiella spp. (22.7%), A. baumannii (11.5 %), P. aeruginosa (10.6%), and other gram-negative bacilli (4.2%, Acinetobacter spp., Proteus mirabilis, Citrobacter spp., Enterobacter cloacae, Alcaligenes spp., Burkholderia cepacia, Neisseria meningitides, Stenotrophomonas maltophilia; Figure 1). Gram-negative organisms had high susceptibility to imipenem (92.4%), meropenem (78.6%), amikacin (76.4%), and gentamicin (72.2%), moderate sensitivity to ciprofloxacin (66.5%), and low susceptibility to cefepime (47.8%), ceftazidime (46.6%), trimethoprim-sulfamethoxazole (45.0%), and cefotaxime/ceftriaxone (42.9%), respectively (Figure 2). The sensitivity of the isolates to meropenem, amikacin, and cefepime was significantly greater in the female population compared with male individuals (86.1% vs. 74.0%; 82.6% vs 72.4% and 56.0% vs 42.5%, respectively). Also, bacterial isolates in the age group under 20 years exhibited higher susceptibility to ciprofloxacin compared to the age group over 20 years (89.5% vs 65.0%).
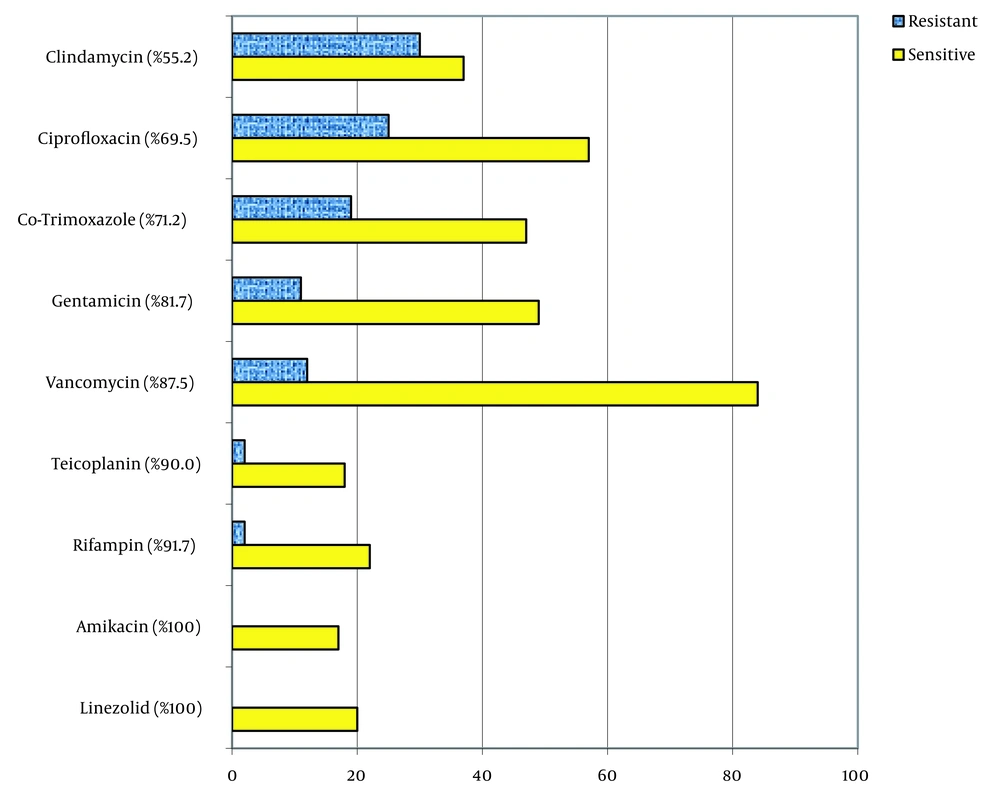
Staphylococcus aureus was the most frequent gram-positive pathogen (71, 14.8%). Other gram-positive isolates included Enterococcus spp. (6.8%) and Streptococcus spp (4.2%). Gram-positive organisms were found to be significantly more prevalent in patients under the age of 20 years (39.6%) compared with those over the age of 20 years (22.8%; P = 0.01), as shown in Figure 1. Gram-positive isolates had high susceptibility to linezolid (100%), amikacin (100%), rifampin (100%), teicoplanin (90%), vancomycin (87.5%), and gentamicin (81.7%), moderate sensitivity to trimethoprim-sulfamethoxazole (71.2%) and ciprofloxacin (69.5%), and low sensitivity to clindamycin (44.8%). The susceptibility of gram-positive organisms to vancomycin was significantly higher in male patients (96.1%) than in female patients (77.8%; P = 0.01), as depicted in Figure 3.
5. Discussion
Our study revealed that the most common cause of community-acquired sepsis was E. coli, Klebsiella spp, S. aureus, Acinetobacter spp, and P. aeruginosa. Gram-positive organisms were found to be more frequently present in individuals under the age of 20 years. Also, we showed that the best antibiotics for empiric therapy of sepsis are a combination of carbapenem (imipenem or meropenem) with linezolid, teicoplanin, or vancomycin. In addition, vancomycin had less antibacterial activity in females.
Our study is the first research that reported the cause and sensitivity of organisms of community-acquired sepsis in the general population. Previous studies have mostly investigated the cause of bloodstream infections without excluding contaminated blood samples or nosocomial sepsis (11, 16, 17, 19) or non-sepsis patients (10, 11, 13, 15-17, 19). The present study excluded non-sepsis cases by recognizing SIRS criteria, contaminated blood samples by discounting a single positive culture of skin flora, and nosocomial sepsis by defined criteria.
Gram-negative bacteria, especially Enterobacteriaceae, were our study's most common cause of sepsis. Escherichia coli was the most common cause of sepsis in our study, followed by Klebsiella spp. Other frequent gram-negative causes of sepsis included Acinetobacter spp and P. aeruginosa. These bacteria were repeatedly reported as the most common causes of bloodstream infections in previous studies (10, 11, 13, 14, 16, 18, 19). In contrast to our study, in endemic areas of malaria disease, Salmonella spp is frequently reported as the cause of bloodstream infections (10, 13, 15). This finding emphasizes the importance of antibiotic coverage for resistant gram-negative organisms in the empiric treatment of septicemic patients (8).
In agreement with previous studies, we found that gram-positive bacteria, especially S. aureus, Enterococcus spp, and Streptococcus spp, were a significant cause of septicemia in the general population (10, 11, 13, 14, 16-18). Also, the prevalence of gram-positive bacteria was significantly higher in the age group of less than 20 years compared to the older age group. This finding is consistent with previous studies (15, 18). This finding emphasizes the significance of combinational empiric therapy of septicemia for coverage of both gram-negative and gram-positive organisms (8). In contrast to our findings, some reports from China and Iran reported a high prevalence of CONS in bloodstream infections (16, 17). However, these bacteria can contaminate blood cultures and cause pseudobacteremia (21). In our study, a single positive culture result of skin flora, such as CONS, was assumed to be contamination and excluded from the final analysis (12, 21).
Our study revealed that gram-negative organisms that cause community-acquired septicemia had a high rate of antimicrobial resistance to extended-spectrum cephalosporins and high susceptibility to carbapenems and aminoglycosides. The isolates showed greater than 90% susceptibility to imipenem and more than 70% sensitivity to meropenem, amikacin, and gentamicin, presenting them as a good candidate for inclusion in the empiric treatment of septicemia in the area. In addition, in the age group of fewer than 20 years, more than 85% of gram negatives were sensitive to ciprofloxacin, and this agent could be a good candidate for early therapy of sepsis in this age group. Nevertheless, alarms about the harm of ciprofloxacin cartilage in animal reports preclude its routine use in children, except in clinical situations in which no response to other antibiotics is achievable (22). Conversely, more than 50 % of gram-negative isolates in our study were resistant to ceftazidime, cefotaxime, ceftriaxone, and cefepime, making them inappropriate for empiric therapy of sepsis in the area. In comparison to our study, a high level of resistance of gram-negative bacteria to cephalosporins and fluoroquinolones and appropriate sensitivity of carbapenems and amikacin were previously reported from a surveillance in China named the China Antimicrobial Resistance Surveillance Trial Program. However, in that study, in contrast to our study, a high level of resistance of gram negatives to gentamicin was reported, which could be due to the difference in antibiotic use in that area (16).
In a study of bloodstream infections in Iran, gram-negative isolates had a very high level of resistance to all examined antibiotics, including extended-spectrum cephalosporins, imipenem, and amikacin. The inclusion of contaminant isolates and nosocomial bloodstream infections in the analysis of that study probably could explain this high level of resistance among gram-negative isolates (17). However, in another study conducted in Iran on pediatric bloodstream infections, similar high levels of susceptibility to ciprofloxacin, amikacin, and gentamicin were observed, along with a high level of resistance to extended-spectrum cephalosporins (19). In one study in Tanzania, similar to our findings, high susceptibility of gram-negative isolates to meropenem and gentamicin was detected. However, in this study, in contrast to our investigation, the high sensitivity of gram-negative isolates to ciprofloxacin and cefotaxime was reported, showing the significance of the geographic area in the selection of antibiotics in the septicemic patient (14).
Gram-positive isolates in our study showed good susceptibility to linezolid, teicoplanin, and vancomycin (making them appropriate antibiotics for inclusion in the empiric treatment of septicemia in the area) and a high level of resistance to clindamycin, ciprofloxacin, and trimethoprim-sulfamethoxazole. In addition, all examined isolates were sensitive to amikacin and rifampin. Nevertheless, despite this high level of susceptibility, aminoglycosides and rifampin should not be applied as a single agent due to the probability of rapid emergence of resistance in these microorganisms (23). A similar high sensitivity of gram positives to linezolid, teicoplanin, vancomycin, rifampin, and aminoglycosides in bloodstream isolates has been previously described from China, but in 1 study in Iran, a high level of resistance to vancomycin and aminoglycosides among gram positives were widely found (17). This difference could be explained by the inclusion of contaminant and nosocomial bloodstream infections in that study (17), which was excluded from our research.
Surprisingly, the susceptibility of gram-positive isolates to vancomycin in our study was significantly lower in females than males. In our research, 96.1% of gram-positive isolates in the male group were sensitive to vancomycin, but in females, sensitivity reached 77.8%. The cause of this difference is unclear, but this finding could signify that linezolid or teicoplanin would be better choices than vancomycin in empiric therapy of sepsis in females.
Our study had several limitations. First, our investigation was performed in 3 referral hospitals, and generalization of the results to all septicemic patients in the area could be biased. Second, there was a lack of clinical data regarding the source of infection and predisposing conditions of the patients. Empirical therapy for septicemic patients in different clinical scenarios could be enhanced by such information. Third, our data were obtained through routine work in the microbiology laboratory, and all isolates were not examined for all antibiotics as some antibiotic disks were unavailable at the time of isolation of microorganisms.
5.1. Conclusions
The high incidence of community-acquired resistant sepsis in the area poses a significant public health concern, particularly when caused by gram-negative bacteria. Our data suggest that third-generation cephalosporins are not effective in treating sepsis in the area. Empiric therapy with a combination of carbapenems and linezolid, teicoplanin, or vancomycin is appropriate before targeted therapy. In addition, our findings suggest that in our area, for females under the age of 20 years, linezolid or teicoplanin may be preferable over vancomycin as part of combination therapy for septicemia.