1. Introduction
The genital system of women can be affected by ascending infections, including sexually transmitted infections (STDs), vaginal flora, and gastrointestinal infections. Pelvic inflammatory disease (PID) is characterized by acute and subclinical infections in the upper genital system, which includes the uterus, fallopian tubes, ovaries, and ligaments. PID can lead to salpingitis, endometritis, oophoritis, perihepatitis, peritonitis, and/or tubo-ovarian abscess (TOA).
TOA typically occurs as a complication of PID. Approximately 200,000 women are admitted annually for PID. It is important to mention that not all cases of TOA are accompanied by PID; however, considering that approximately one-third of hospitalized PID cases are found to have TOA.
The incidence of TOA may be increasing, although the cause of this increase is not clear. It could be related to changing treatment for PID. Currently, hospitalized patients have severe disease or TOA. Patients with TOA are usually between the ages of 15 and 40 years, although age should not exclude the diagnosis.
2. Case Presentation
A 22-year-old Afghan woman was admitted to Firoozabadi Hospital of Iran University of Medical Sciences with severe generalized abdominal pain, fever, chills, anorexia, and nausea. She had undergone a normal vaginal delivery (NVD) and had an episiotomy performed 17 days ago due to preterm premature rupture of membranes (PPROM) at 35 weeks of pregnancy. Her labor was induced using oxytocin. On the 5th and 16th days after NVD, she experienced green vaginal discharge and fever, prompting her to seek medical attention at a general hospital. She underwent a COVID-19 test and was also advised to have a complete blood count (CBC) and abdominal ultrasound.
On the 17th day, she was referred for further evaluation. During the examination, her temperature was recorded as 39°C, her pulse rate (PR) was 144 bpm, her blood pressure (BP) was 70/40 mm/Hg, and her respiratory rate (RR) was 45 bpm. Her episiotomy incision site had not healed, and she exhibited generalized tenderness and rebound tenderness in her abdomen. Pelvic examination revealed severe bilateral tenderness. Consequently, two intravenous lines were established, and a bladder catheter was inserted. Various tests were ordered, including CBC, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), fibrinogen (Fib), blood culture × 3, urine culture, blood urea nitrogen (BUN), and creatinine (Cr) levels were assessed. She received 1 liter of normal saline and was initiated on intravenous meropenem 1 g three times a day and vancomycin 1 g twice a day. However, her urine output was nonexistent, leading to emergency pelvic and abdominal ultrasounds.
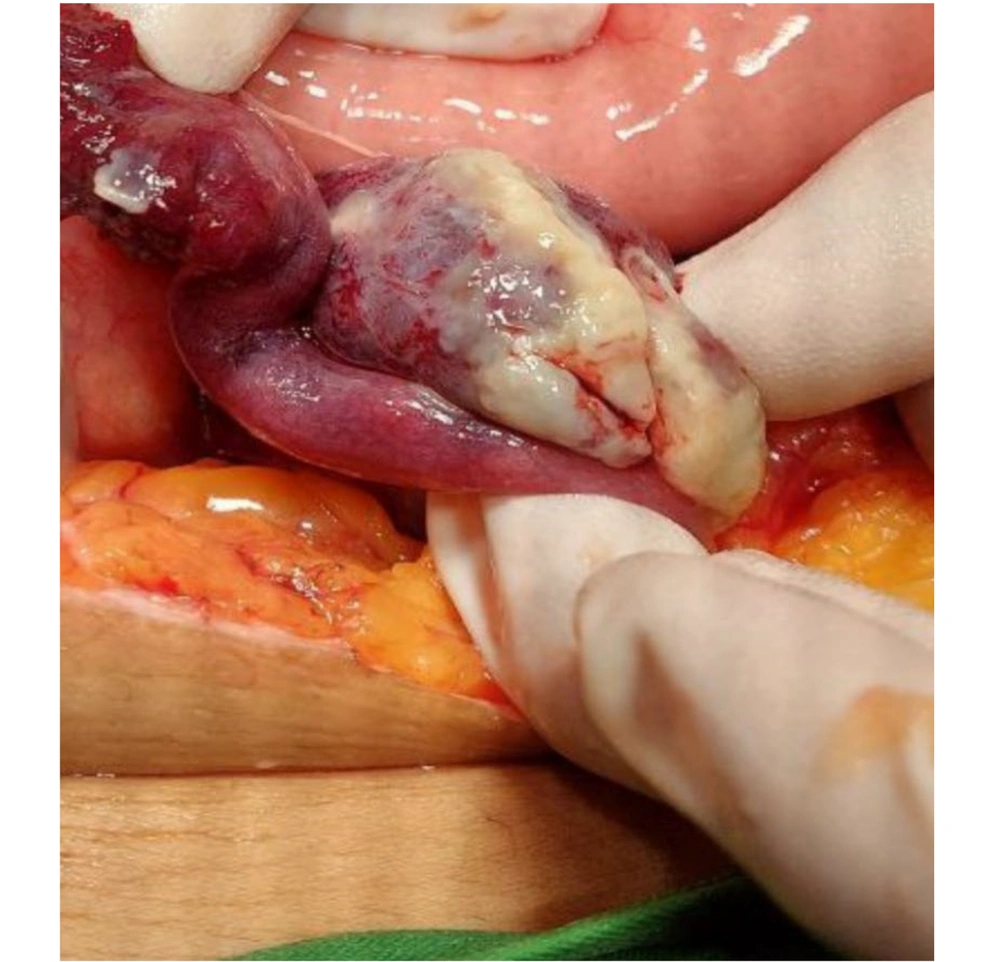
The ultrasound examination revealed the presence of free fluid in the pelvic and paracolic grooves and enlargement of the right ovary, with a normal appendix, kidney, liver, and spleen. With informed consent from the patient, emergency surgery under general anesthesia was chosen as the course of action. During the procedure, a midline incision was made, and approximately 500 - 600 cc of green pus fluid was aspirated from the abdomen. An exploration of the pelvis and abdomen exposed a right TOA. Ten milliliters of pus were sent for bacteriology and mycology testing, and examinations of the stomach, bowels, spleen, liver, and pancreas revealed no abnormalities. A perforated TOA was identified on the right ovary (Figure 1) and pus in the anterior and posterior cul-de-sac. The large uterus and left ovary appeared normal. Following this discovery, a right adnexectomy was performed while preserving the uterus and left adnexa. The intra-peritoneal cavity was thoroughly irrigated with 3 liters of warm normal saline, and a corrugated drain was placed in the pelvic region. Subsequently, the patient was transferred to the intensive care unit (ICU), where a treatment regimen of antibiotics, heparin (5000 units twice a day), and painkillers was initiated.
In the ICU, the patient’s vital signs were recorded as follows: BP of 80/40, heart rate (HR) of 130 bpm, RR of 20 bpm, and a temperature of 37.3°C. Basic laboratory analysis indicated a hemoglobin (HB) level of 8.3, a platelet count (PLT) of 243,000, a white blood cell count (WBC) of 29,000 with neutrophils comprising 95%, and normal PT, partial PTT, and INR. The Fib level was 420, Cr measured at 1.3, liver function tests (SGOT-SGPT, ALP, and bilirubin) were within the normal range, while CRP and ESR were elevated, measuring 75 and 70, respectively. Consultations were sought from infectious diseases and cardiology specialists, who added caspofungin (50 g daily) and metoprolol (25 g three times a day), respectively. Additionally, one unit of packed red blood cells was transfused.
Echocardiography revealed normal findings. In the ICU, the patient exhibited a urine output of 100 cc/h, and the drainage output was 500 cc/24 h. By the first day, her BP had improved to 95/60 mm/Hg, PR measured at 104 bpm, RR was 25 bpm, oxygen saturation (O2Sat) reached 98% with oxygen supplementation, and the woman displayed no signs of toxicity or illness.
On the second day, her WBC was 25,000, with neutrophils at 90%. HB was 9.7, platelet levels were within the normal range, CRP was 65, and creatinine was 0.7. A chest X-ray was conducted due to a decline in O2Sat, revealing right pleural effusion. A pleural fluid tap was performed, and the bacteriology results returned as normal. On the second day, the patient was able to consume food orally, and the bladder catheter was removed. Vital signs remained stable, and blood analysis indicated a trend toward normal levels.
By the third day, the WBC had decreased to 21,000, while liver function tests, creatinine, blood urea nitrogen, prothrombin time, partial thromboplastin time, and international normalized ratio were all within normal limits. Blood culture results showed Staphylococcus epidermidis with sensitivity to Amikacin, Vancomycin, and Ciprofloxacin, while the urine culture was negative. The patient's condition remained stable.
On the fourth day, the WBC further decreased to 15,000, with neutrophils comprising 70%. HB levels were at 10, and the remaining blood analysis parameters were normal. No fungal growth was observed in the abdominal pus, leading to the discontinuation of caspofungin. The COVID-19 test yielded a negative result.
On the fifth day, the patient was transferred to the surgery ward with a BP of 105/74 mm/Hg, PR of 98 bpm, RR of 18 bpm, temperature of 37°C, and O2Sat of 95% without the need for oxygen supplementation. On the seventh day of hospitalization, a second blood culture showed no bacteriological growth, and the drain was removed.
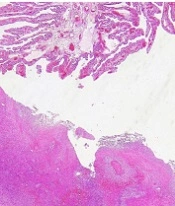
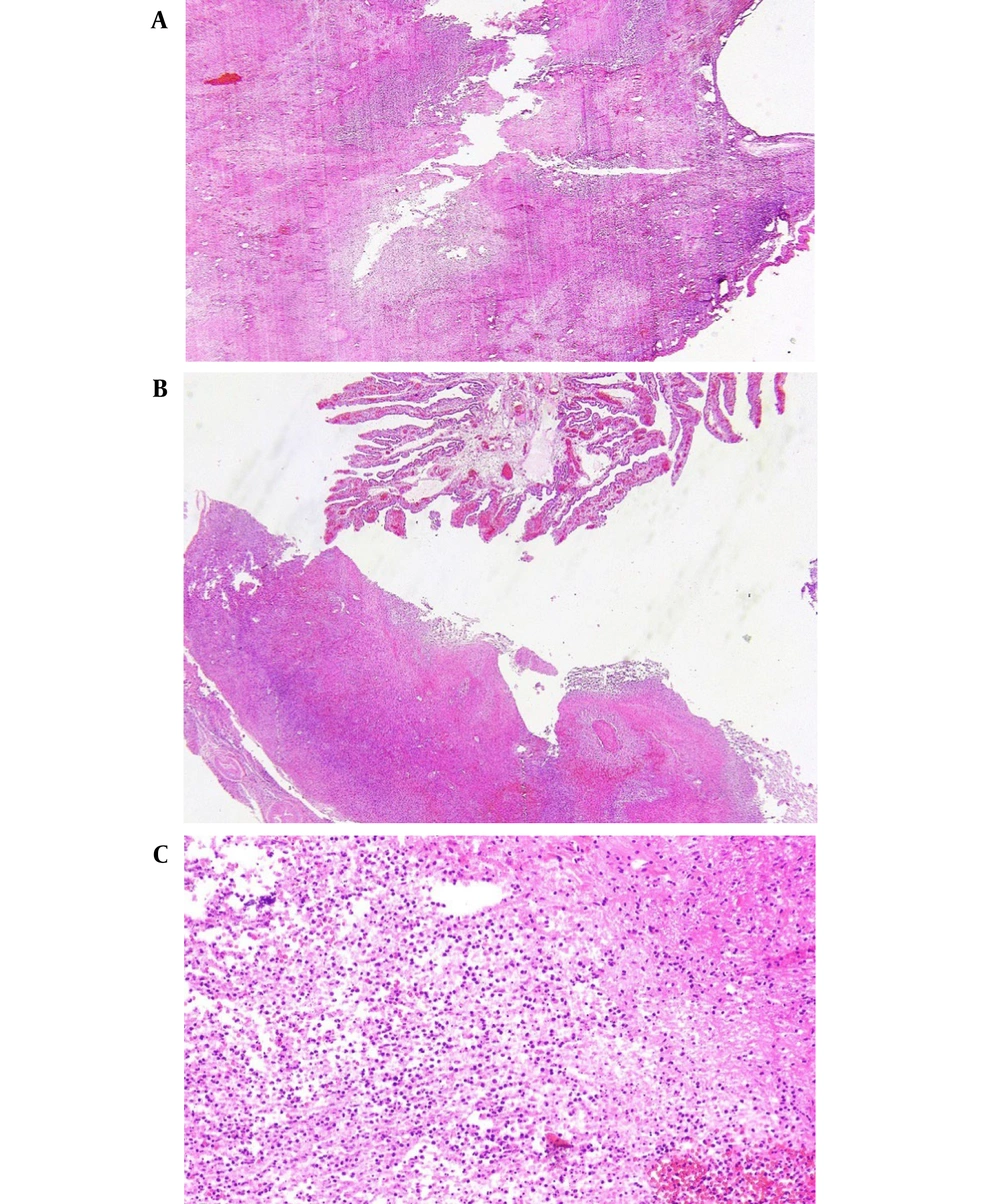
Finally, on the 9th day, the WBC was 7,700, with neutrophils at 65%. HB measured 10 mm/Hg, PLT was 600,000, creatinine was 0.6, liver function tests were normal, and prothrombin time, partial thromboplastin time, and international normalized ratio were all within normal limits. The second blood culture revealed no bacteriological growth. After 7 days of hospitalization, the woman was discharged in good condition with prescriptions for ciprofloxacin 500 mg three times a day, doxycycline 100 mg twice a day, Co-amoxiclav three times a day, and enoxaparin 40 mg every other day. She had a follow-up appointment at the gynecology clinic one week later, and her CBC was within normal limits. The pathology report confirmed the presence of a TOA (Figure 2A-C).
A TOA is a life-threatening disease requiring medical and/or surgical treatment, and rupture of the abscess can lead to sepsis and septic shock. The mortality rate for TOA was approximately 50% before the discovery of broad-spectrum antibiotics and surgical interventions (1, 2). Mortality rates are not reported for ruptured abscesses in the literature (1, 3, 4).
Risk factors for TOA are similar to those for PID and include having multiple sexual partners, a history of PID, and oocyte retrieval for in vitro fertilization (5-7). The use of an IUD has been considered a risk factor for the development of TOA, specifically unilateral TOA, but this association has not been conclusively proven by multiple studies (8-11). The elevated risk of developing PID among women appears to be limited to the first three weeks after IUD insertion.
Some literature suggests that women with HIV infection are more likely to develop a TOA than non-HIV-infected women (12, 13).
The mechanism of TOA formation has not been fully explained and may result from PID, infection associated with the bowel, appendicitis, adnexal surgery, or hematologic spread.
TOAs can be classified into either tubo-ovarian complexes (an agglutination of pelvic organs with or without bowel involvement) or collections of pus that can be drained. Different treatments are required depending on these two forms (e.g., drainage is typically feasible only for collections) (8, 14). Additionally, worsening infection may lead to sepsis.
The bacteria found in TOAs are similar to those in uncomplicated PID (15), and mixtures of aerobic, facultative, and anaerobic organisms have been isolated from TOAs (14, 16). Common organisms include Escherichia coli, Bacteroides fragilis, Prevotella, aerobic streptococci, and Peptostreptococcus (16). Occasionally, unusual pathogens, including Pasteurella multocida, Salmonella species, Candida species, and Streptococcus pneumoniae, have been identified (17-20).
Neisseria gonorrhoeae and Chlamydia trachomatis are rarely isolated from TOAs. The role of these organisms appears to be limited to cervicitis or PID (16). Some literature suggests that N. gonorrhoeae facilitates the invasion of the upper genital tract by lower genital tract flora (21).
However, the presentation of some patients differs from classic TOAs. Fever may not be present in all patients, and some may only report low-grade nocturnal fevers, chills, diffuse persistent upper abdominal pain, or changes in bowel habits.
A "ruptured" TOA refers to an abscess that has started to leak inflammatory contents into the abdominal cavity, necessitating immediate surgery. Approximately 15% of TOA patients present with signs and symptoms suggestive of a ruptured TOA. In such cases, surgical evaluation is recommended.
The presentation of an ovarian abscess is uncommon in gynecological cases. Over the last 110 years, only 120 cases of ovarian abscesses have been reported in the literature. In our study, the participant had a history of NVD and episiotomy without previous PID. She presented with generalized abdominal pain, vaginal discharge, fever, chills, and septic shock. Immediate laparotomy and adnexectomy were performed, and her pathology report confirmed TOA. She received broad-spectrum antibiotics for 10 days and recovered. The woman was discharged with oral antibiotics.
3. Conclusions
TOA after NVD is a rare occurrence and can develop in patients with PPROM. Hospitalization and treatment with broad-spectrum antibiotics or drainage can prevent rupture and emergency surgery, along with other life-threatening complications.