1. Background
Postpartum depression (PPD) poses a significant mental health challenge for women, manifesting as a form of depression occurring during pregnancy or within four weeks after childbirth (1). As per the International Classification of Diseases 10 (ICD-10), PPD is characterized by persistent low mood, feelings of sadness, or loss of interest, alongside poor concentration, disrupted appetite, and feelings of worthlessness, typically starting four weeks post-delivery (1, 2).
Although various brain regions, such as the hippocampus, nucleus accumbens, anterior cingulate gyrus, amygdala, prefrontal cortex, and lateral habenula, regulate emotions, the hippocampus garners particular attention due to its role in cognition and memory function (3). Given its rapid oxygen consumption, high levels of easily oxidizable polyunsaturated fatty acids, and low antioxidant levels, hippocampal neurons are particularly susceptible to oxidative stress (4). Studies have shown decreased activity of antioxidant enzymes and increased oxidative stress markers in the hippocampus of individuals with depression (5). Many individuals experiencing depression also exhibit cognitive impairments, including difficulties with focus, memory, and stress tolerance (6). Pharmacologically induced oxidative stress has been associated with anxiety-like behavior and memory deficits in rats, suggesting a potential causal link (7). However, antioxidant treatment has been shown to mitigate both behaviors, suggesting a potential therapeutic approach (8). Research on individuals with severe depression has confirmed the role of reactive oxygen species in the pathogenesis of depression. The action mechanism likely involves the hyperactivity of the HPA axis (9). Studies have shown that cognitive deficits are associated with elevated cortisol levels, and several cognitive measures improve with the reduction of cortisol production (10). Long-term exposure to glucocorticoids can lead to neurodegeneration, cognitive impairments, anxiety, and depressive-like behavior in the hippocampus (11), in addition to promoting cell death and reducing hippocampal volume (12).
Selective serotonin reuptake inhibitors (SSRIs) represent the primary treatment for PPD (13), highlighting the need for effective and more affordable alternatives to antidepressant prescriptions. Quercus, a traditional herbal remedy, thrives in dry and low-temperature climates. It is part of the Fagaceae family, specifically within the genus Quercus, which includes about 500 species (14). This genus is known for containing tannins, gallic and ellagic acids, vitamins C, A, and B, various galloyl and hexahydroxydiphenoyl derivatives, linoleic and linolenic acids, polymeric proanthocyanidins, proteins, minerals, and fatty acids. The Quercus brantii fruit also comprises several secondary metabolites. The antioxidative properties of these natural compounds stand out as their most significant biological feature (15). To date, the effects of the hydro-alcoholic extract and oil of Q. brantii on memory deficits and hippocampal oxidative stress profiles in the PPD model have not been reported
2. Objectives
This study aimed to evaluate the effectiveness of a hydro-alcoholic extract and oil from Q. brantii fruit on spatial memory deficits and hippocampal oxidative stress in the PPD model induced by progesterone.
3. Methods
3.1. Extract Preparation and Characterization
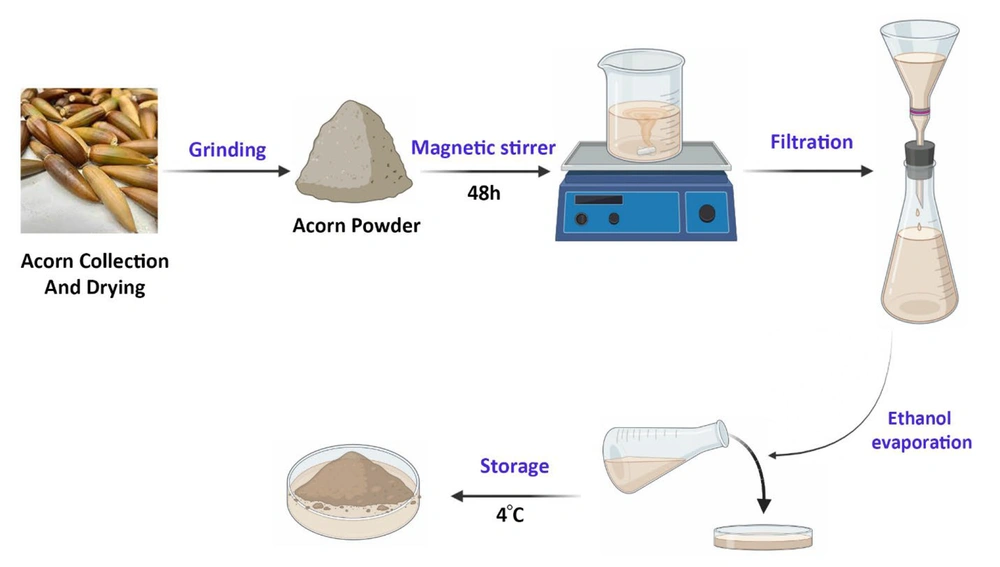
The oak acorn (Q. brantii) was collected in August 2021 from the verdant forests near Khorramabad city, located in Lorestan province, Iran. A sample weighing 100 grams was ground into a fine powder and then soaked in 70% ethanol for 48 hours at room temperature to prepare the alcoholic extract (16). This extract was then filtered using Whatman No. 1 filter paper to the greatest extent possible, and the solvent was evaporated to yield a concentrated extract. Subsequently, the extract samples were stored in universal bottles and kept refrigerated at 4°C until their use was required (Figure 1). The acorn oil was acquired from the Roghankadeh company in Tehran, Iran.
For an experimental study, a total of 42 adult female Wistar rats (3 months old) weighing between 200 - 250 g were sourced from the animal facility at the Faculty of Veterinary Medicine in Ahvaz, Iran. These rats were housed in a regulated environment maintained at 24 ± 1°C with a relative humidity of 45 - 55% and had free access to water and standard rat chow. Furthermore, the rats experienced a 12-hour light/dark cycle. After a period of acclimatization, vaginal swabs were collected from all the rats to determine those in a normal estrus cycle. For the subsequent experiments, only rats that exhibited a normal estrus cycle were selected (17).
3.2. Experimental Design and Components
3.2.1. Animals Grouping
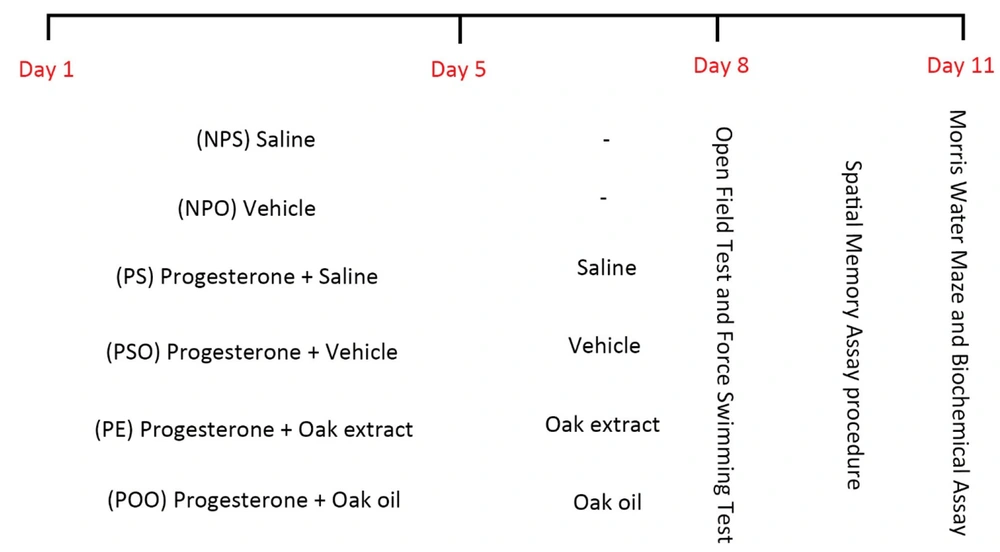
A total of 42 animals were randomly assigned into six groups (n = 7 each). These groups included a control group, a progesterone withdrawal (PWD) group, and groups treated with oak extract and oil, as depicted in Figure 2. Each treatment group received oak extract and oil in a single dose for eight consecutive days, culminating in an open field, forced swimming tests, and a Morris water maze assessment on the eighth day (18). All administrations were carried out via intraperitoneal injections.
3.2.2. Induction of the Postpartum Depression Model
The study by Beckley and Finn (19) followed the protocol for PPD modeling. The PWD protocol entailed administering daily injections of progesterone (5 mg/kg) (Iran Hormone Company, Tehran, Iran), dissolved in sesame oil (Roghankadeh Company, Tehran, Iran) and stirred overnight at 37°C for 5 days. After this 5-day regimen, progesterone was withdrawn for 3 days (20).
3.2.3. Open Field Test
Locomotor activity assessment was performed using open field tests (OFT) at the procedure's conclusion. At the beginning of the test, rats were placed at the center of an 80x80x40 cm cuboid area, which had interior surfaces coated in black. A rat was considered to have crossed a line when all four of its feet were within a single square (21).
3.2.4. Forced Swimming Test
The forced swimming test (FST), incorporating slight modifications to the techniques of Lucki (22), was employed to assess depression-like behavior in animals. For this study, female rats were gently placed into crystal-clear, transparent glass cylinders measuring 60 cm in height and 25 cm in diameter. These cylinders were filled with water maintained at a temperature of 25 ± 2°C, with the water level set at 30 cm. The entire test lasted 6 minutes, during which the behaviors of the rats were meticulously recorded with a video camera (23).
3.2.5. Morris Water Maze Test
On the eighth day, following the completion of the treatment phase, the Morris water maze (MWM) test was conducted to evaluate the rats' capacity for learning and memory retention (24). The apparatus consisted of a circular tank made of high-quality stainless steel, black in color, with a diameter of 180 cm and a height of 50 cm. The tank was filled with water to a depth of 25 cm, which was kept at a constant temperature of 25 ± 1°C. The maze was geographically divided into four quadrants: Northeast, Northwest, Southeast, and Southwest. Surrounding the pool, four visual markers of different sizes and colors served as distant cues. A circular platform was strategically placed in the center of the northwest quadrant, painted in the darkest black to signify its role as an escape mechanism. This platform was submerged in the water maze, with just 1 cm visible above the water surface. Additionally, the water was dyed black with a non-toxic dye to camouflage the escape platform.
For three consecutive days, each rat underwent four training trials per day to learn how to escape. In each trial, rats were given a total of four attempts, with 10-minute intervals between each attempt each day, to locate the hidden platform in the water from each of the pool's four cardinal points (Northeast, Northwest, Southeast, and Southwest). If a rat could not find the platform within a 1-minute training session, the investigator gently guided it to the goal. Once the rats located the platform, they were allowed to remain on the concealed platform for twenty seconds. During the test phase, the hidden platform was removed from the water. To assess their spatial memory, the rats were permitted to swim freely in the pool for sixty seconds. The swim paths of the animals in each trial were automatically recorded by a video camera. Offline analysis was conducted to calculate the distance traveled, escape latency, swimming speed, and the time spent in the target quadrant.
3.2.6. Hippocampal Sample Preparations
On the 11th day, after completing the behavioral tests, the rats were euthanized. The animals underwent deep anesthesia, and their brains were rapidly removed, with the hippocampal tissues being carefully isolated on ice kept at 2 - 4°C. The hippocampi were precisely weighed before being homogenized in cold PBS to achieve a 10% (w/v) homogenate solution, thereby minimizing contamination from peripheral blood. After homogenization, the mixture was centrifuged at 10 000 rpm for six minutes at 4°C. The supernatant obtained was meticulously collected, and aliquots were stored at -80°C for subsequent analyses of oxidant-antioxidant parameters, vitamin D, and corticosterone levels.
3.3. Biochemical Assays
The levels of reduced glutathione (GSH), catalase, superoxide dismutase (SOD), and malondialdehyde (MDA) in the hippocampus were analyzed using a state-of-the-art UV/Vis spectrophotometer (Optimize, South Korea). The concentration of GSH was determined using the reaction between thiols and Ellman's reagent (DTNB), serving as a substrate. This method involves evaluating the absorption of visible light at 412 nm, based on Ellman's technique (25).
Catalase activity was assessed using the method developed by Koroliuk et al. The procedure began by adding 0.1 mL of homogenate to 2 mL of a 0.03% hydrogen peroxide solution, followed by the addition of 50 μL of 4% ammonium molybdate to halt the reaction. The intensity of the resulting color was measured at a wavelength of 410 nm relative to a blank (26).
SOD activity was quantified by measuring the inhibition of nitro blue tetrazolium (NBT) reduction in the presence of SOD. The intensity of the color produced was measured at 560 nm, according to the methodology described by Kono (27).
The levels of MDA were investigated using the thiobarbituric acid reactive substances (TBARS) method developed by Placer et al. (28). TBARS measurement was performed with a spectrophotometer set to 532 nm.
Vitamin D and corticosterone levels were evaluated following the standard protocols provided with the Monobind kit (USA, kit number: 9425-300 for Vitamin D and 3625-300 for corticosterone). To measure vitamin D levels, 100 µL of Vitamin D Releasing Agent was mixed into 25 µL of the sample and incubated at room temperature for 30 minutes. The mixture was then treated with 350 µL of buffer, and its absorbance at 450 nm was recorded. For corticosterone measurement using the Monobind kit, 25 µL of the sample was mixed with 50 µL of Cortisol Enzyme Reagent and 50 µL of Cortisol Biotin Reagent and incubated at room temperature for 60 minutes. After adding 300µL of wash buffer and 100µL of working substrate solution, the absorbance in each well was determined at 450 nm using the Microplate Reader.
3.4. Statistical Analysis
The Graph Pad InStat software (version 3.00) was used for comparing the mean data across different groups, with the significance level set at P < 0.05. The data were presented as mean ± SEM for visualization. The analysis included a one-way analysis of variance (ANOVA), followed by Tukey's post hoc statistical tests for comparisons among multiple groups. For comparisons between the two groups, the student's t-test was applied.
4. Results
4.1. Effects of Hydro-Alcoholic Oak Extract and Oak Oil on Locomotor Activity in the OFT of PPD
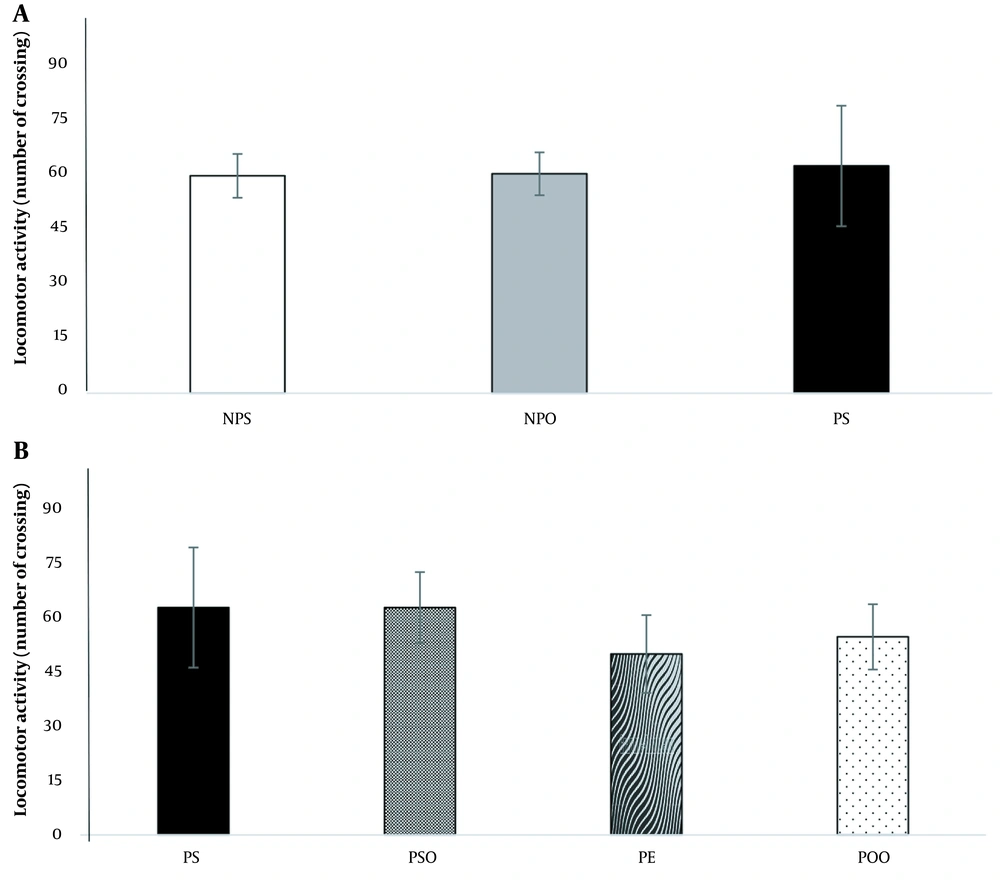
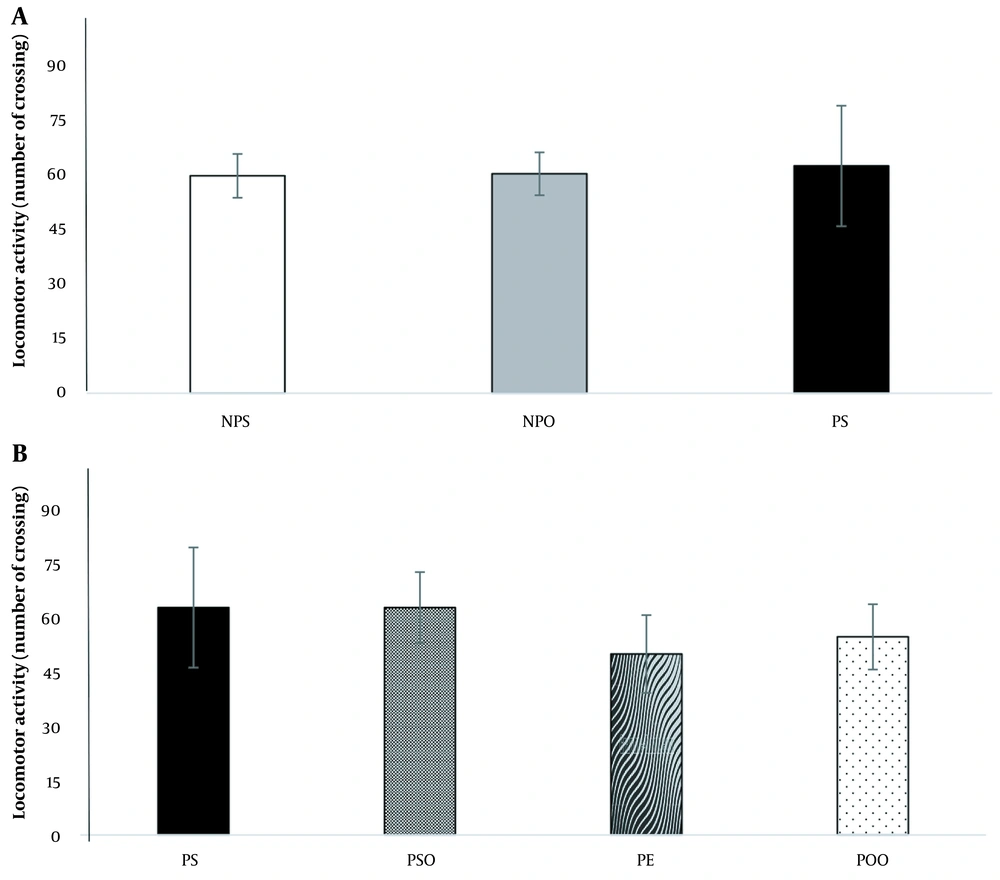
The analysis results indicated no significant difference between the depression and control groups in the average number of line crossings in the OFT (Figure 3A). Additionally, neither oak oil (45%) nor hydro-alcoholic oak extract (100 mg/kg) demonstrated a stimulatory effect on the mobility of rats (Figure 3B).
The effect of the induced PPD model and hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) on PPD on the OFT. NPO: No PPD + sesame oil; NPS: No PPD + saline; OFT: Open field tests; PE: PPD + oak extract; POO: PPD + oak oil; PPD: Postpartum depression; PS: PPD + saline; PSO: PPD + sesame oil. Data are expressed as mean ± SEM.
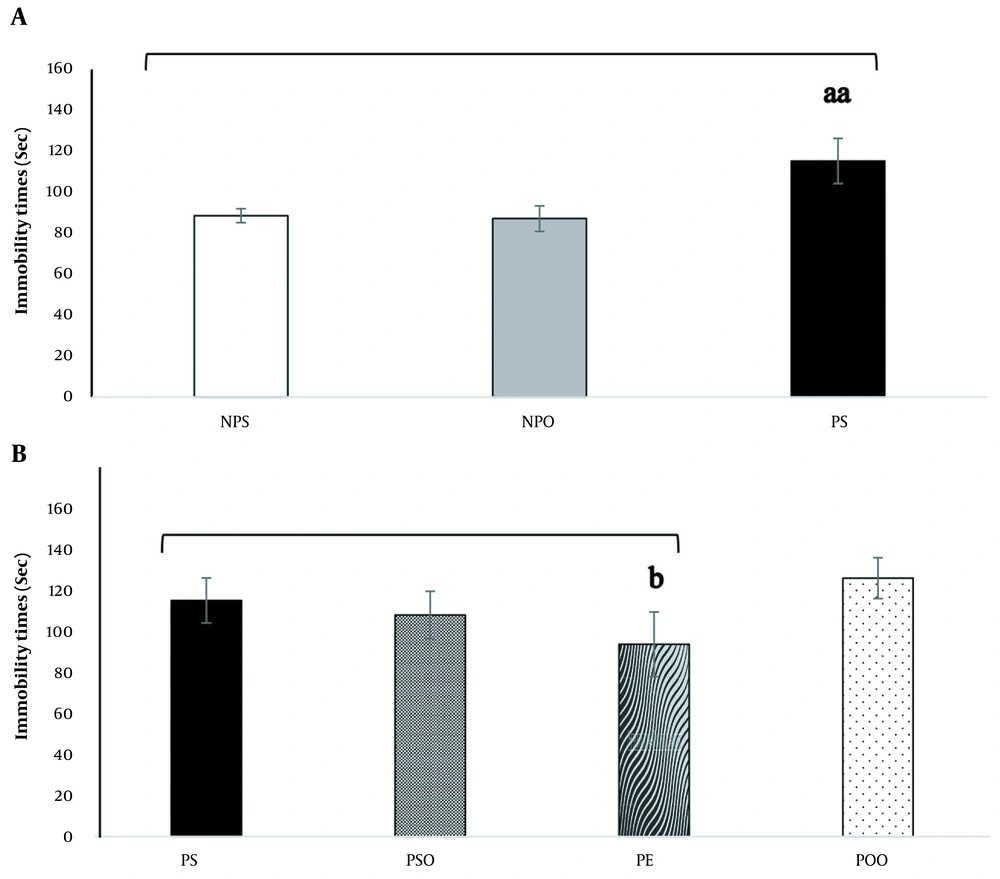
4.2. Effect of Hydro-Alcoholic Oak Extract and Oak Oil on the FST and PPD
The impact of administering hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) on the FST in a progesterone-induced PPD model is depicted in Figure 4. There was a significant difference in total immobility time between groups, with the depressed group showing higher total immobility times compared to the NPS and NPO groups. However, this effect was not observed in rats treated with hydro-alcoholic oak extract (100 mg/kg) (P < 0.001) (Figure 4A). The administration of hydro-alcoholic oak extract (100 mg/kg) significantly reduced immobility time compared to the PS group (P < 0.05). Interestingly, the application of oak oil (45%) did not result in a significant change compared to the PS group (P < 0.05) (Figure 4B).
The impact of the induced PPD model and the administration of hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) on the PPD in the forced swimming test (FST). Significant differences between groups in (A), are denoted by "aa" (P < 0.001), and significant differences between groups in (B), are indicated by "b" (P < 0.05). FST: Forced swimming test; NPO: No PPD + sesame oil; NPS: No PPD + saline; PE: PPD + oak extract; POO: PPD + oak oil; PPD: Postpartum depression; PS: PPD + saline; PSO: PPD + sesame oil. The data are expressed as mean ± SEM.
4.3. Morris Water Maze Test
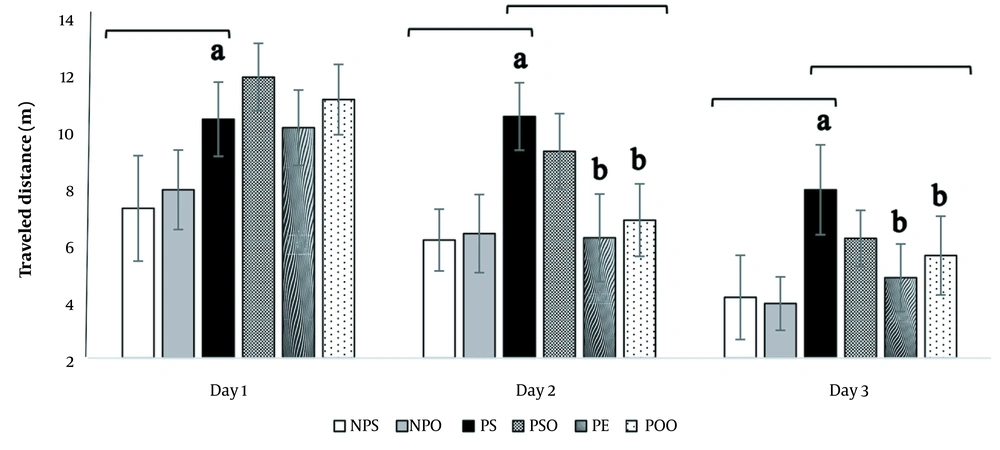
4.3.1. Traveled Distance
The distance traveled to locate the platform is an essential measure in the MWM test that reflects the learning process. The results indicate a significant difference between the treatment and depression groups in terms of the distance traveled scores over the three-day learning phase (Figure 5). The administration of progesterone increased the traveled distance (P < 0.05), whereas the hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) significantly decreased the traveled distance (P < 0.05).
The effects of hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) on the traveled distance in spatial memory impairment in the PPD model. Significant differences between the PS, NPS, and NPO groups are marked by "a" (P < 0.05), and significant differences between the PS, PSO, PE, and POO groups are denoted by "b" (P < 0.05). NPS: No PPD + saline; NPO: No PPD + sesame oil; PPD: Postpartum depression; PS: PPD + saline; PSO: PPD + sesame oil; PE: PPD + oak extract; POO: PPD + oak oil. The data are presented as mean ± SEM.
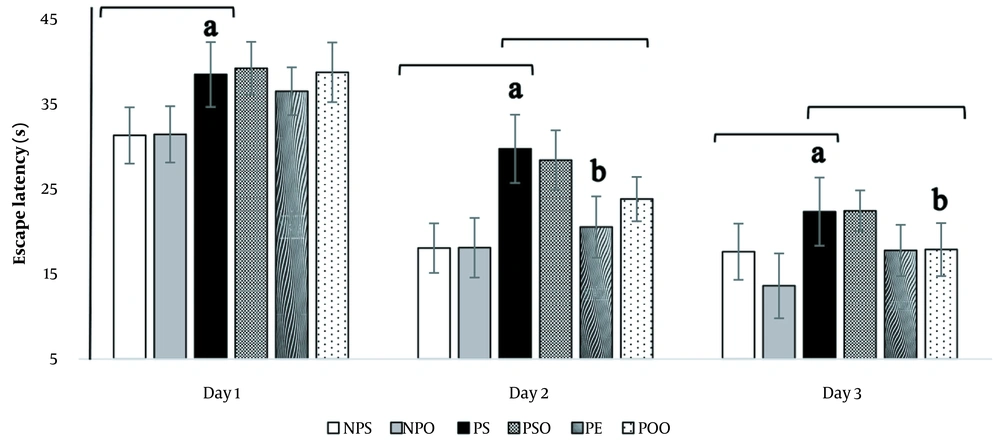
4.3.2. Escape Latency
This study aimed to explore the impact of various interventions on the escape latency of rats during a three-day learning period. Escape latency, defined as the time it takes for rats to locate the target platform, was evaluated using repeated measures ANOVA. The findings showed a significant difference in escape latency between the depressed and treatment groups. Specifically, rats subjected to intraperitoneal (i.p.) injections of progesterone displayed a notable increase in escape latency (P < 0.05), whereas those treated with hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) experienced a significant reduction in escape latency (P < 0.05) (Figure 6).
The influence of hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) on escape latency in spatial memory impairment within the PPD model. Significant differences between the PS, NPS, and NPO groups are highlighted by "a" (P < 0.05), and significant differences between the PS, PSO, PE, and POO groups are marked by "b" (P < 0.05). NPS: No PPD + saline; NPO: No PPD + sesame oil; PPD: Postpartum depression; PS: PPD + saline; PSO: PPD + sesame oil; PE: PPD + oak extract; POO: PPD + oak oil. Data are presented as mean ± SEM.
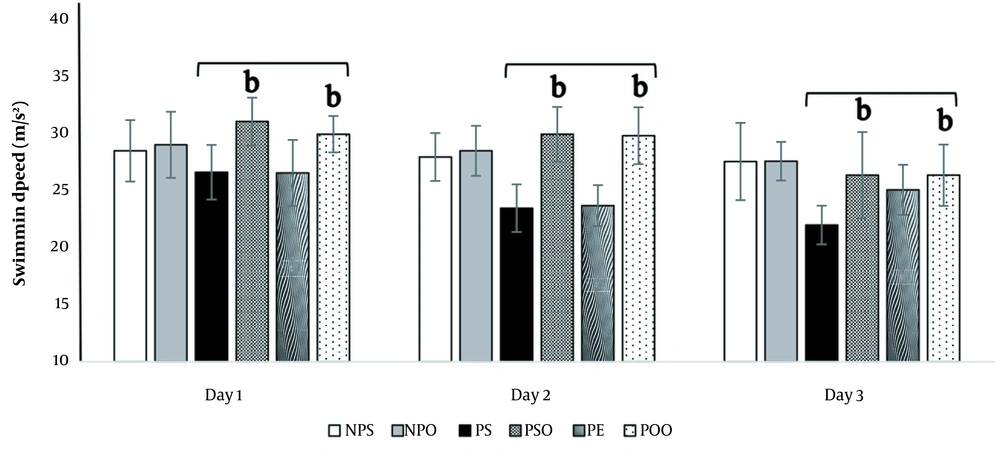
4.3.3. Swimming Speed
The analysis revealed a variance in the average swimming speed between the depressed and treatment groups. The induction of PPD led to a decrease in the swimming speed of the rats, though this decrease was not statistically significant (P < 0.05) (Figure 7). However, treatment of the depressed rats with hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) resulted in a significant enhancement of their swimming speed in comparison to the PS group (P < 0.05) (Figure 7).
The effects of hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) on swimming speed in spatial memory impairment within the PPD model. Significant differences between the PS, PSO, PE, and POO groups are marked by "b" (P < 0.05). NPS: No PPD + saline; NPO: No PPD + sesame oil; PPD: Postpartum depression; PS: PPD + saline; PSO: PPD + sesame oil; PE: PPD + oak extract; POO: PPD + oak oil. The data are presented as mean ± SEM.
4.3.4. Time Spent in the Target Quadrant
The evaluation of time spent in the target quadrant was conducted on the fourth day of the MWM test. The depressed group showed a reduced duration in the target quadrant, though this reduction was not statistically significant (P < 0.05) (Figure 8A). On the other hand, rats treated with hydro-alcoholic oak extract (100 mg/kg) demonstrated a statistically significant increase in time spent in the target quadrant compared to the PS group (P < 0.05) (Figure 8B).
The impact of the induced PPD model and treatment with hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) on the duration spent in the target quadrant in the PPD model. Significant differences between groups in (B) are indicated by "b" (P < 0.05). NPO: No PPD + sesame oil; NPS: No PPD + saline; PE: PPD + oak extract; POO: PPD + oak oil; PPD: Postpartum depression; PS: PPD + saline; PSO: PPD + sesame oil. The data are expressed as mean ± SEM.
4.4. Effects of Hydro-alcoholic Oak Extract and Oil on Biochemical Factors
Statistical analysis revealed that progesterone administration resulted in a significant decrease (P < 0.05) in the average GSH level in the hippocampal region compared to the NPS group (P < 0.05). Furthermore, the administration of hydro-alcoholic oak extract (100 mg/kg) significantly increased (P < 0.05) the average GSH level in the hippocampal region, while oak oil (45%) did not significantly affect GSH levels (P < 0.05) (Table 1).
| Groups | GSH, µmol/mL | SOD, U/mL | CAT, µmol/min | MDA, nmol/mL | Vit D, ng/mL | Corticosterone, nmol/mL |
|---|---|---|---|---|---|---|
| NPS | 0.451 ± 0.05 | 0.108 ± 0.02 | 0.325 ± 0.03 | 0.293 ± 0.07 | 2.23 ± 0.27 | 0.85 ± 0.01 |
| NPO | 0.433 ± 0.06 | 0.103 ± 0.04 | 0.304 ± 0.02 | 0.39 ± 0.15 | 2.11 ± 0.13 | 0.94 ± 0.08 |
| PS | 0.354 ± 0.05a | 0.90 ± 0.03a | 0.281 ± 0.01a | 0.74 ± 0.01aa | 1.58 ± 0.16a | 1.41 ± 0.14a |
| PSO | 0.383 ± 0.04 | 0.106 ± 0.02 | 0.271 ± 0.02 | 0.573 ± 0.09b | 1.99 ± 0.22 | 1.11 ± 0.09 |
| PE | 0.428 ± 0.07b | 0.182 ± 0.02bb | 0.302 ± 0.02b | 0.329 ± 0.1bb | 2.16 ± 0.19b | 1.22 ± 0.08 |
| POO | 0.37 ± 0.04 | 0.114 ± 0.01bb | 0.29 ± 0.03 | 0.444 ± 0.1bb | 2.38 ± 0.17b | 1.12 ± 0.01 |
Abbreviations: NPS: No PPD + saline; NPO: no PPD + sesame oil; PPD: Postpartum depression; PS: PPD + saline; PSO: PPD + sesame oil; PE: PPD + oak extract; POO: PPD + oak oil.
a Data are presented as mean ± SEM.
b The levels of GSH, SOD, CAT, and VitD were significantly decreased (P < 0.05) in the depression induction model compared to the control, while the levels of MDA (P < 0.001) and corticosterone (P < 0.05) increased significantly. In contrast, the administration of hydroalcoholic oak extract (100 mg/kg) significantly elevated the levels of GSH (P < 0.05), SOD (P < 0.001), CAT (P < 0.05), and VitD (p < 0.05), and significantly reduced the level of MDA (P < 0.001). Corticosterone levels were not significantly altered by the oak extract (100 mg/kg). When compared to the depressed group, the administration of oak oil (45%) significantly raised the levels of SOD (P < 0.001) and vitamin D (P < 0.05), and significantly lowered the level of MDA (P < 0.001). The effects of oak oil on catalase, GSH, and corticosterone levels were not statistically significant. Significant differences between the PS, NPS, and NPO groups are indicated by "a" (P < 0.05) and "aa" (P < 0.001), and significant differences between the PS, PSO, PE, and POO groups are marked by "b" (P < 0.05) and "bb" (P < 0.001).
The data revealed a significant difference (P < 0.05) in hippocampal catalase levels between the PS group and the NPS and NPO groups. However, catalase levels were significantly elevated in the group treated with hydro-alcoholic oak extract (100 mg/kg) compared to the PS group (P < 0.05). Conversely, oak oil (45%) did not exhibit any effect on catalase levels.
The findings demonstrate that the depressed groups exhibited significantly lower levels of hippocampal SOD compared to the NPS groups (P < 0.05). Conversely, the levels of SOD in the groups treated with hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) were significantly higher than those in the PS group (P < 0.001).
The statistical analysis highlighted a significant increase in the mean MDA level in the hippocampus of the PS group (P < 0.001) due to progesterone withdrawal (PWD). Compared to the PS group, the administration of hydro-alcoholic oak extract (100 mg/kg) led to a decrease in MDA levels. Similarly, the use of oak oil (45%) resulted in a significant reduction in MDA levels compared to the control group (P < 0.001).
The analysis also showed that progesterone administration significantly reduced the average vitamin D level in the hippocampal region compared to the NPS group (P < 0.05). Furthermore, the administration of hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) significantly increased the mean vitamin D level in the hippocampal region (P < 0.05).
Additionally, the data indicated that the depression-experiencing groups had significantly higher levels of hippocampal corticosterone compared to the NPS groups (P < 0.05). However, while the hydro-alcoholic oak extract (100 mg/kg) and oak oil (45%) were observed to decrease corticosterone levels, this decrease was not statistically significant (P < 0.05).
5. Discussion
This study demonstrated that prolonged withdrawal from progesterone therapy could induce symptoms of depression in rats. Moreover, it is the first to investigate the effects of Q. brantii oil and hydro-alcoholic extract on memory impairment and the oxidant-antioxidant balance in the hippocampus within a PPD model. Our research reveals that creating a PPD model in rats alters the levels of vitamins and hormones, including corticosterone and vitamin D, in the hippocampus. It also leads to an increase in oxidant factors like MDA and a decrease in antioxidant levels such as SOD, GSH, and catalase. Individually, these changes are capable of causing the memory impairment observed in depressed rats.
In this investigation, the creation of a PPD model involved the chronic intraperitoneal administration of progesterone for 5 days, followed by a 3-day cessation, and this approach is supported by previous studies (21, 29, 30). Research involving both humans and animals has demonstrated the hippocampus's high sensitivity to stress (31, 32). The connection between oxidative stress and depression is well-documented (33, 34). Experimental evidence has shown that increased brain lipid peroxidation and other factors, such as elevated levels of nitric oxide and cyclooxygenase-2 (COX-2) activity, contribute to heightened oxidative stress (35), thus corroborating our findings. In this study, a significant increase in MDA was observed in the PPD rat model, potentially leading to hippocampal damage and subsequent memory impairment.
Furthermore, studies have revealed that administering vitamin D3 to mice mitigates the oxidative stress and depressive-like behavior triggered by repeated corticosterone injections (36). Supplementation with vitamin D3 lowers lipid peroxidation, protein carbonyl content, and nitrite levels, which may contribute to alleviating symptoms of depression (37). Our results indicate that vitamin D is a crucial factor in addressing PPD-related memory deficits. We noted a significant reduction in vitamin D levels in the depressed groups, whereas the administration of oak extract and oak oil significantly increased vitamin D concentration in the hippocampus. Additionally, a key observation from this study is that rats showing depressive symptoms had elevated corticosterone levels compared to those without such symptoms, underscoring the significant role of this hormone in the development of depression and the associated memory impairment.
In the MWM test, mice lacking extracellular SOD did not show a significant increase in the time spent exploring the target quadrant over three days of training (38). In our study, we observed a reduction in SOD enzyme levels in the hippocampus of depressed rats.
Acorn, known for its traditional medicinal properties, exhibits antioxidative capabilities (39). To our knowledge, this study is the first to reveal the protective effects of Q. brantii against memory impairment in a PPD model. Consistent with our findings, a related study demonstrated that using Q. brantii extract led to a reduction in MDA levels in mice exposed to lead (Pb), aligning with the observations made by Dogan et al. (39). Carotenoids have been identified as having the ability to mitigate neuroinflammation and oxidative damage induced by corticosterone, indicating a potential antidepressant effect (40). The antioxidant activity of Quercus extract is attributed to gallic and ellagic acids. Castalagin, vescalagin, and roburin are examples of ellagitannins, which are prominent phenolic compounds found in Quercus species (15). In rat liver tissue, the intake of gallic acid was shown to increase levels of glutathione, GSH peroxidase, GSH reductase, and GSH S-transferase while reducing oxidative stress and the content of oxidized glutathione (GSSG). Therefore, the application of gallic acid effectively inhibits oxidative stress in rats subjected to a high-fat diet (HFD) (41).
Ceribasi et al. showed that cyclophosphamide-induced abnormalities in sperm, elevated plasma MDA levels, and decreased erythrocyte SOD activity; it also reduced CAT activity, caused necrosis, led to the production of immature germ cells, resulted in testicular atrophy, and caused congestion. However, these effects were alleviated by treatment with ellagic acid, demonstrating its protective properties (42). Moreover, phenolic compounds can chelate transition metals and scavenge metal ions that promote damage through free radicals. The hydrophobic phenyl rings and phenolic hydroxyl groups in phenolic structures enhance the formation of hydrogen-bonding interactions. These interactions could also benefit various proteins, including cytochrome P450 isoforms, cyclooxygenases, lipoxygenases, and xanthine oxidases, potentially inhibiting oxidative enzymes responsible for radical generation (43).
In some cases, the effects observed from oak oil were less pronounced than those from the extract, possibly due to its higher fat content and lower dosage. To date, no research has been conducted on the effects of oak oil on rats, leaving a gap in the available information. Therefore, future studies could explore various dosages of the oil or isolate and test a specific lipid.
5.1. Conclusions
In conclusion, this study suggests that the antidepressant effect of Q. brantii extract and oil is linked to the stimulation of neurotrophic factors and the restoration of oxidative stress balance, indicating potential neuroprotective benefits. Thus, Q. brantii extract and oil present themselves as viable options for the treatment of memory impairment in PPD.