1. Background
Minerals and trace elements (MTEs), often referred to as minor elements, are inorganic components found in plant and animal foods (1). These minerals are typically categorized into two main groups based on their recommended daily intake: micro-minerals and macro-minerals (2). Micro-minerals include ferric iron (Fe), zinc (Zn), iodine (I), Mn, chromium (Cr), copper (Cu), molybdenum (Mo), silicon (Si), aluminum (Al), arsenic (As), tin (Sn), lithium (Li), and nickel (Ni). The ionized forms of trace elements play a crucial role in various essential biological functions. They act as co-factors in metalloenzymes, such as arginase (which requires Mn) and glutathione peroxidase (which requires magnesium (Mg)). They also serve as enzymatic co-factors in cuproenzymes and possess antioxidant properties, as seen in ceruloplasmin (which requires Cu). Additionally, trace elements like Fe and Zn are vital for the formation of heme proteins such as hemoglobin and myoglobin and for maintaining the structural integrity of antioxidant enzymes like superoxide dismutase. However, the dynamics and biochemical processing of these elements vary; selenium (Se) is highly absorbed, unlike Mn. Se and Zn are primarily excreted through urine, whereas Mn is mainly eliminated through feces (3, 4).
The concentrations of MTEs are usually regulated by strict homeostatic mechanisms (5). Emerging evidence suggests that exercise and post-exercise recovery lead to changes in the homeostasis of tissue and serum levels of MTEs due to excretion through sweat and urine (5). Moreover, it has been observed that exercise-induced mobilization of micro-elements from the bloodstream to metabolic organs such as the liver and erythrocytes, as well as excretion through sweat and urine, takes place (6). Conversely, a deficiency in MTEs has adverse effects, especially in tissues requiring large amounts, such as skeletal muscle. This is because the musculoskeletal system contains the largest proportion of some MTE reserves, such as Zn and calcium (5). Furthermore, impairments in MTEs are associated with a decrease in maximum oxygen consumption (VO2max), which could potentially affect athletic performance in various scenarios involving high metabolic demands during physical exercise (7). The research conducted by Maynar et al. revealed a reduction in both serum and urine Zn levels among top endurance athletes after 6 months of training (8). Consequently, these athletes exhibit lower levels of Mg and phosphorus (P) in their blood compared to sedentary individuals (9). Notably, when athletes were categorized based on the type of metabolic activity they engaged in, the blood concentration of Mg was found to be higher in aerobic athletes than in anaerobic or mixed aerobic-anaerobic athletes (9). Conversely, a study indicated that athletes had higher blood levels of trace elements compared to control groups (10).
Many studies (11-13) have recommended the use of herbal supplements to restore trace mineral element (TME) losses during or after exercise due to their non-pharmacological effects and nutraceutical properties. Cucurbita pepo, commonly known as 'Pumpkin,' is a fruit that has recently been recognized for its benefits. It is grown at various altitudes, from sea level to high elevations. Pumpkin is known for its low-fat content and seeds rich in protein. Additionally, it possesses anti-inflammatory and antioxidative properties, along with hepatoprotective actions (11). This fruit is rich in essential macro and micro-minerals such as phosphorus, Mg, calcium, manganese, Cu, and Zn, which play a crucial role in replenishing the minerals lost from the body. Moreover, pumpkin seeds are a rich source of both essential and non-essential amino acids, including lysine and glutamic acid, which are vital for various physical and mental processes (11). Studies have indicated that pumpkin seeds with a higher iron content facilitate the binding of heme and globin components, which is crucial in low oxygen situations during intense physical exercise. The numerous benefits of pumpkin seeds have intrigued experts, who are now exploring the idea of using this mineral-rich crop as an herbal medical supplement for various conditions that disrupt homeostasis (14).
Exercise initiates a coordinated series of physiological responses and alters metabolism, providing an excellent opportunity to explore the metabo-circulatory link between hormones, energy balance, and the selective utilization of minerals and trace elements (MTEs). Biological adaptations associated with chronic physical activity are thought to play a crucial role in the mobilization of TMEs. However, despite significant advances in understanding the complex etiology of trace element dynamics in tissues and body fluids during and after exercise and recognizing pumpkin seed oil's compensatory role for exercise-induced TME deficiency, many aspects concerning the down-regulation signals responsible for mineral replenishment remain unclear. To address this gap, we hypothesized that pumpkin seed oil supplementation would benefit TME homeostasis following chronic physical activity.
2. Objectives
Our study aimed to compare the effects of aerobic exercise and pumpkin seed oil supplementation on the dynamics of TMEs in the blood, liver, and gastrocnemius muscles of male Wistar rats.
3. Methods
3.1. Ethical Approval
The standards for animal care and ethics were set according to the Guide for the Care and Use of Laboratory Animals (15), which was approved by the Ethics Committee of the University of Mazandaran (IR.UMZ.REC.1402.036).
3.2. Animals
Forty male Wistar rats, weighing between 130 to 150 g and aged 8 to 10 weeks, were obtained from the Razi Animal Institute's Center at Karaj Medical University in Karaj City, located in Alborz Province, Iran. The rats were housed in the animal room of our faculty at 24 ± 1°C, with 45% to 55% humidity and a 12:12 light/dark cycle. They had access to food and water ad libitum before and throughout the experiment. Upon arrival at our laboratory animal experiment site, the rats were allowed one week to acclimate to their new surroundings, with unrestricted access to water and a standard diet composed of 63.9% mixed carbohydrates, 20.3% protein, and 15% lipid matter. After this period, the rodents were randomly divided into four groups of 10 rats each: (1) Saline control group (SC), (2) training at 25m/min group (ST), (3) pumpkin seed oil supplementation group (PC), and (4) training at 25m/min + pumpkin seed oil supplementation (PT). Body weight and food intake were recorded weekly.
3.3. Exercise Programs and Load Measurements
After a week of adapting to treadmill running (three times a week, 10-minute sessions, 0% incline), the training groups began steady-state running, which included 60 minutes of running on a motorized treadmill (designed by Prof. Abbass Ghanbari-Niaki, Faculty of Sports, University of Mazandaran, Mazandaran, Iran, featuring a fourteen-lane Iranian Model) at a speed of 25 meters per minute, five days a week for six weeks. Each training session also included a 10-minute warm-up and cool-down period (the speed was set to 10 m/min at 0% incline). The training protocols were adapted from previous studies (16, 17), where a speed of 25 m/min elicited approximately 65% of VO2Peak (18). The rats underwent the exercise training protocol during the active phase of their day (the dark phase). To mitigate cage stress, the sedentary rodents were isolated for the same duration on a stationary treadmill as the training groups.
3.4. Pumpkin Seed Oil Supplementation
3.4.1. Extraction
In summary, the pumpkin seeds were finely ground and placed into a decanter funnel containing a 50% water-to-hexane solvent ratio. After allowing the solvent to contact the pumpkin seeds for 12 hours, the oil phase of the solvent and water were separated from the container. The oil-depleted residue was then transferred to a percolator for extraction purposes. The required amount of solvent (60-degree alcohol) was poured over the oiled material to fully cover its surface. The solvent was left in contact with the pumpkin seeds for a duration of 72 hours. The hydroalcoholic extract was then separated by filtering the mixture through sterile paper at a 1:1 ratio, while the solvent was removed using a rotary evaporator at a low temperature (below 60°C) (19). The results of the inductively coupled plasma optical emission spectrometry (ICP-OES) analysis for the trace elements content of the pumpkin seed oil used in the present study are summarized in Table 1.
| Trace Mineral Elements (TMEs), mg/L | Cu | Fe | Mg | Mn | Zn |
|---|---|---|---|---|---|
| Cucurbita pepo seed oil | 0.04 | 0.88 | 13.86 | 0.06 | 0.22 |
3.4.2. Supplementation Dosage
The effective dosage of the concentrated extract was determined by weighing it relative to the weight of the rodents (4 ml/kg/day) over a period of eight weeks via oral gavage. The control groups were likewise administered saline in a similar manner and at the same determined ratio to standardize the gavage-induced stress. Weekly re-weighing was conducted to obtain the precise dosage per kilogram of body weight (20, 21).
3.5. Blood and Tissue Collection
Following a 6-week treatment period, rats that had been fasting for 12 hours received an intraperitoneal injection of a mixture of ketamine and xylazine (at doses of 80 and 12 mg/kilogram, respectively) 48 hours after their last exercise session. Cardiac blood samples were collected and centrifuged at a speed of 2 500 revolutions/minute for 15 minutes at a temperature of 4°C. The resulting plasma was stored at -20°C until TME tests were performed. Liver and gastrocnemius tissues were excised, immediately frozen at -80°C until they were processed, and weighed for TME analysis.
3.6. TMEs Assay
3.6.1. Digestion
Gastrocnemius muscle and liver tissues, as well as serum samples, were prepared for Zn, Fe (Fe), manganese Mn, Mg, and Cu analysis using a method adapted from Kechrid and Bouzerna (22). To prepare the samples for wet tissue digestion, 0.5g of gastrocnemius and liver tissues were weighed and digested with a hot mixture of nitric acid and perchloric acid (2:1; 1 mL) until the tissue was completely disintegrated. The resulting solutions were diluted with double-distilled water to a final volume of 25 mL and filtered using filter paper (Whatman®, ashless, grade 42). Trace elements were then measured using the ICP-OES (Optima 5300 DV, USA) technique (23).
3.7. Statistical Analysis
Each value was expressed as a mean ± SEM. Groups were compared using one-way ANOVA, followed by the Bonferroni post-hoc test. The significance level was set at P < 0.05. Statistical analysis was conducted using SPSS 22.0.
4. Results
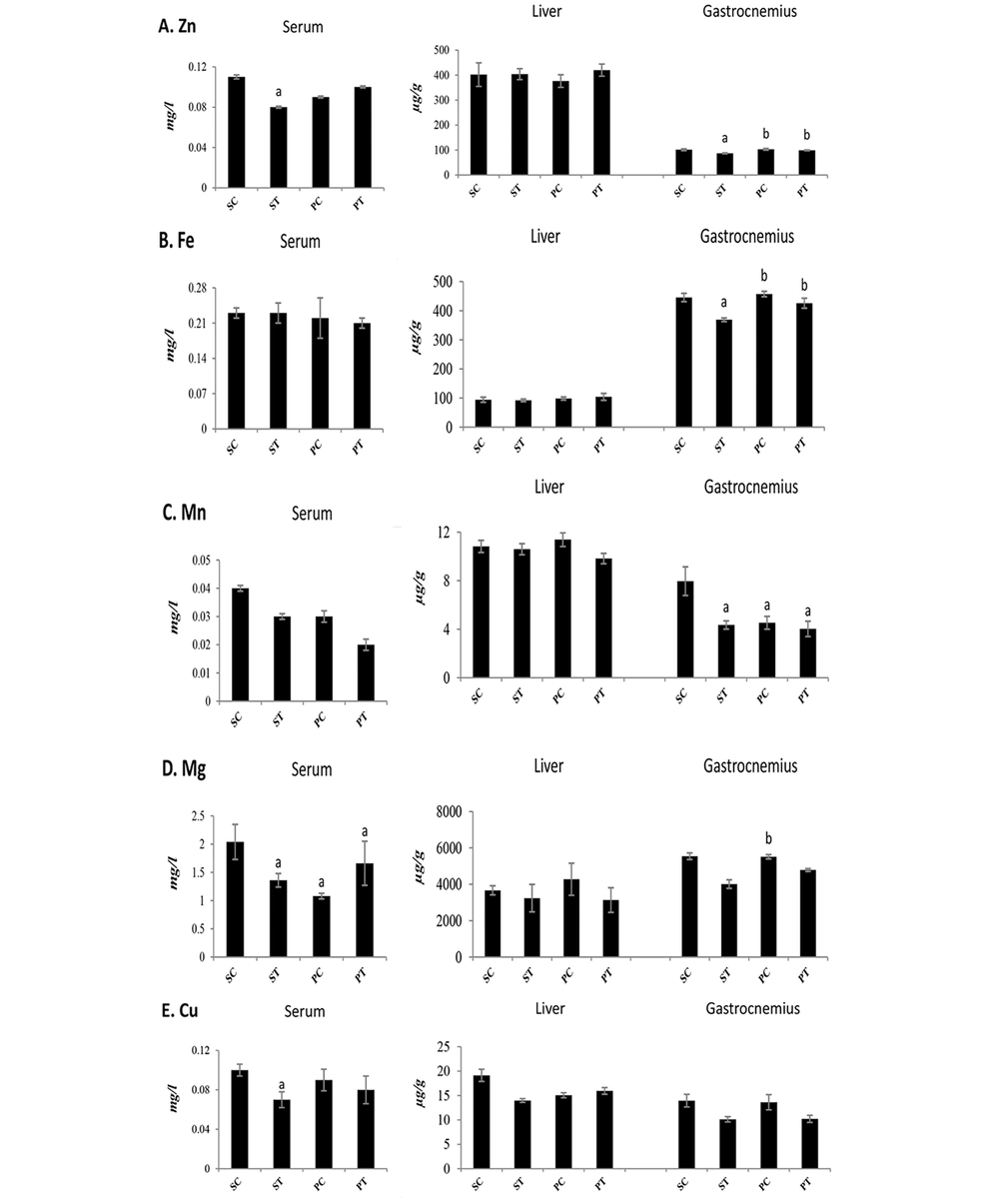
One-way ANOVA results showed that the combination of physical exercise and pumpkin seed oil intake for six weeks significantly altered blood levels of Mg (P = 0.026) and gastrocnemius concentrations of Zn (P = 0.002), Mn (P = 0.048), Mg (P = 0.018), and liver concentrations of Cu (P = 0.001) among the study groups (Table 2).
| Trace Mineral Elements and Sample | Saline Control | Saline Training | Pumpkin Seed Oil | Training + Pumpkin Seed Oil | P-Value |
|---|---|---|---|---|---|
| Zn | |||||
| Serum, mg/L | 0.11 ± 0.002 | 0.08 ± 0.001 | 0.09 ± 0.001 | 0.1 ± 0.001 | 0.043 b |
| Liver, µg/g | 94.23 ± 8.74 | 92.12 ± 4.61 | 98.23 ± 5.4 | 104.09 ± 11.96 | 0.633 |
| Gastrocnemius, µg/g | 101.4 ± 3.7 | 86.62 ± 1.88 | 102.9 ± 3.59 | 98.76 ± 1.7 | 0.024 b |
| Fe | |||||
| Serum, mg/L | 0.23 ± 0.01 | 0.23 ± 0.02 | 0.22 ± 0.09 | 0.21 ± 0.01 | 0.728 |
| Liver, µg/g | 402.08 ± 47.21 | 403.89 ± 22.02 | 376.36 ± 25.18 | 420.08 ± 24.43 | 0.512 |
| Gastrocnemius, µg/g | 445.42 ± 14.24 | 368.89 ± 6.27 | 457.36 ± 9.36 | 425.74 ± 17.11 | 0.037 b |
| Mn | |||||
| Serum, mg/L | 0.04 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.002 | 0.02 ± 0.002 | 0.52 |
| Liver, µg/g | 10.83 ± 0.5 | 10.6 ± 0.46 | 11.39 ± 0.57 | 9.83 ± 0.43 | 0.194 |
| Gastrocnemius, µg/g | 7.96 ± 1.18 | 4.35 ± 0.34 | 4.53 ± 0.53 | 4.03 ± 0.63 | 0.048 b |
| Mg | |||||
| Serum, mg/L | 2.04 ± 0.31 | 1.36 ± 0.12 | 1.08 ± 0.05 | 1.66 ± 0.39 | 0.026 b |
| Liver, µg/g | 3367.81 ± 250.59 | 3239.04 ± 752.64 | 4277.46 ± 882.03 | 3134.52 ± 676.96 | 0.156 |
| Gastrocnemius, µg/g | 5543.28 ± 180 | 4010.32 ± 235 | 5509.64 ± 116 | 4785.28 ± 76 | 0.032 b |
| Cu | |||||
| Serum, mg/L | 0.1 ± 0.056 | 0.07 ± 0.008 | 0.09 ± 0.011 | 0.08 ± 0.014 | 0.001 b |
| Liver, µg/g | 19.14 ± 1.24 | 13.98 ± 0.37 | 15.06 ± 0.51 | 15.95 ± 0.68 | 0.711 |
| Gastrocnemius, µg/g | 13.95 ± 1.3 | 10.13 ± 0.54 | 13.62 ± 3.26 | 10.22 ± 0.73 | 0.907 |
a Values are expressed as mean ± standard error.
b A significant group difference at P < 0.05 based on ANOVA.
The Bonferroni post-hoc test results demonstrated that 6 weeks of supplementation with pumpkin seed oil, an effective ergogenic aid, increased the concentrations of Zn, Fe, Mn, and Mg in the gastrocnemius tissue as well as the levels of Mg in the serum when compared to the control group (P < 0.05). However, other tissue or serum samples did not show alterations in response to pumpkin supplementation or physical exercise (Figure 1). Overall, pumpkin seed oil supplementation up-regulated and replenished the exercise-induced excretion of TMEs from the liver, gastrocnemius, and body fluids in healthy rats (Figure 1).
5. Discussion
According to reports, the physiological characteristics that most distinctly differentiate efficient performance from fatigue status are the concentrations of TMEs in body fluids and metabolic tissues (6). In particular, serum and gastrocnemius sources of TMEs are important physiological indicators of performance during physical activity (6, 24). This study is the first to investigate the compensatory impacts of pumpkin seed oil supplementation on serum, liver, and gastrocnemius levels of TMEs in male rats in response to physical exercise over 6 weeks of the study protocol. We hypothesized that pumpkin seed oil supplementation would lead to greater improvements in TME resources of serum, liver, and gastrocnemius compared to the saline control group in response to exercise-induced TME excretion. Major research findings derived from ICP-OES results support our hypothesis, with increased uptake of TMEs from serum and compensatory effects of pumpkin supplementation.
5.1. Serum, Liver, and Gastrocnemius Concentrations of TMEs in Response to Moderate-Intensity Training
The metabolism of trace elements may be influenced by several factors, including exercise. Regular physical training can increase the demand for essential trace elements, either by accelerating their breakdown or by reducing their excretion from the body. Recent reports suggest that physical training leads to changes in the levels of certain biological minerals in the blood during exercise and recovery (25). Our study's results indicate a continuous decrease in serum Mg and Zn levels, as well as in Zn, Mn, and Fe levels in the gastrocnemius muscle, after six weeks of moderate-intensity physical activity, with the statistical significance of these findings indicated by a P-value of less than 0.05. Zn, which accounts for 60% of the body's total content in muscle tissue and 30% in bones, may experience variations in concentration in specific areas during exercise and the subsequent recovery period, leading to changes in local levels. Especially during inflammatory processes, such as stress and post-traumatic conditions in athletes, serum Zn levels decrease as Zn is reallocated to repair damaged tissues (26). Zn is absorbed by the liver, kidney, and spleen through the stimulation of metallothionein production, but its levels decrease in erythrocytes, bones, muscles, and urine (27). Moreover, Rodriguez Tuya et al. observed that anaerobic athletes (e.g., judo and fencing participants) had higher blood Zn levels compared to aerobic athletes (e.g., cyclists and endurance sports participants), attributing this to the reduced Zn mobilization resulting from impaired energy metabolism and antioxidant activities in anaerobic exercise (28). The decrease in blood Zn levels, but not in the liver, observed in this study may be related to the previously mentioned factor. Zn's antioxidant properties are credited to its role in superoxide dismutase (SOD), its ability to stabilize cell membranes, protect protein thiol groups from oxidation, and compete with Cu and Fe for oxygen binding, which reduces the generation of hydroxyl radicals (OH•) from membrane phospholipids (29). Training appears to increase the mRNA levels of SOD in aerobic organs such as the liver, heart, and deep muscles like the vastus lateralis (29). In addition, significant correlations have been observed between trace element (TE) levels and the antioxidant system, indicating that TEs impact antioxidant enzymes and vice versa. This suggests that the formation of exercise-induced reactive oxygen species (ROS) and the corresponding antioxidant responses are interconnected (29, 30). Key trace elements, such as Zn and Cu, play a crucial role in these antioxidant processes. They act as cofactors in important antioxidant enzymes, such as glutathione peroxidase (GPx) and Zn- Cu superoxide dismutase (Zn-Cu SOD), which help reduce oxidative stress (OS) caused by ROS. This may explain the observed decrease in Zn levels in the serum and gastrocnemius muscle following submaximal physical exercise, as implemented in the current study (31). Guo et al. found negative correlations between the levels of Cu in the blood and the amounts of Cu -Zn superoxide dismutase (Cu-Zn SOD) (32). It has also been proposed that the elimination of Zn, Mg, and Cu occurs through sweat and urine (32). Additionally, an inverse correlation exists between Zn and Mg levels and interleukin-6 (IL-6) (33). Athletes with higher levels of IL-6 tend to have lower Zn levels in their blood and experience increased Zn loss through sweat (7). This can result in an increased influx of Zn into the liver and red blood cells, potentially due to an increase in metallothionein, which contributes to the sequestration of Zn in the liver, leading to a decrease in circulating Zn during the recovery phase of exercise (33). Furthermore, training might cause changes in gastrointestinal function, potentially affecting the absorption and excretion of Zn and Mg (4). Research consistently shows that there is a significant increase in the amount of Zn and Mg excreted in urine (about 50%) on the day following continuous exercise compared to the day before without exercise (5). Prolonged exercise has been shown to result in an increase in urinary Zn loss of about 1 mg per day, which is approximately 6% of the required daily Zn intake (28). Furthermore, quantifying the depletion of trace minerals through sweat excretion presents challenges due to analytical obstacles, such as the very low levels of minerals and technological difficulties in accurately collecting whole-body sweat samples. Additionally, variations in mineral levels are found in sweat across different regions (32). The total amount of Zn and Mg lost from the whole body surface without exercise has been estimated at approximately 0.8 mg per day (28). Considering a sweating loss of 2 liters and a total body sweating concentration of 600 picograms per liter, the amount of Zn and Mg lost through the skin during exercise could potentially reach 1.5 milligrams. Consequently, the amount of Zn lost from the surface may account for up to 10% of the required daily intake of Zn (30). Unfortunately, this research did not measure the quantity of sweating and excretion rate due to the challenges associated with assessment. The study found that 23% of elite runners had Zn concentrations below 11.5 pmol/L, the lower limit of the normal range for Zn concentrations. The authors of the study suggested an inverse relationship between serum Zn concentration and training mileage (34). The lack of changes in liver Zn levels may be attributed to simultaneous increases in plasma volume and Zn-carrier proteins, which are likely to result in decreased serum Zn concentrations and a lower ratio of Zn to albumin protein (35). It has been proposed that the release of Zn and Mg during exercise is influenced by the overall status of total metal elements (TMEs) in the body (26-32, 35). Moreover, the specific nature of aerobic exercise prompts Zn and Mg to enter red blood cells (RBCs) as a mechanism for the body to adapt to moderate physical activity. This is another potential explanation for the observed decrease in serum TME levels after exercise (30, 31).
Following the conclusion of the exercise session, a series of events unfold, the most significant of which is the initiation of inflammatory processes. This involves the secretion of cytokines and the infiltration of immune cells into the active muscles (36). Our theory posits that the decrease in Zn levels in the blood within 24 - 48 hours after ceasing exercise is due to inflammation and associated mechanisms triggered by exercise-induced muscle injury. Consequently, we observed a decline in the levels of Zn and Manganese in the gastrocnemius muscle. Research indicates that inflammatory cytokines can cause significant changes in the levels of Zn transporters and metallothioneins. These molecules play a pivotal role in regulating the balance of Zn and manganese within cells, thus ensuring their stability (36). The presence of inflammatory cytokines during the initial phase of the body's response to injury, coupled with changes in the pressure that draws fluid into tissues after aerobic exercise, can be accounted for by the movement of Zn and Mg from the bloodstream into the interstitial spaces and the liver. This phenomenon adheres to a two-compartment model of kinetics for tissue metal elements during the recovery period following exercise (5).
The current investigation found that serum levels of Cu were significantly lower compared to the saline control group (P < 0.05). According to a study by Holloszy in 1967, the decrease in serum Cu concentration following prolonged physical exercise can be attributed to the increased activity of cytochrome C oxidase, which is enhanced after endurance training. This increased utilization of Cu helps to maintain the activity of cytochrome C oxidase. The suggested relationship indicates that lower levels of Cu in the blood serum may be associated with decreased concentrations of Cu in red blood cells. This correlation is thought to be linked to the role of cuproenzymes in antioxidant activities, especially in extracellular compartments (37). A limitation of the current study is the lack of measurement of TMEs in erythrocytes, which would provide a more complete understanding of TME dynamics. The varied observations regarding the impact of exercise on TME dynamics may be due to the energy system responsible for supplying ATP during physical activity, as well as the intensity and duration of the exercise (26). Similarly, in the study of TME chemistry, competitive interactions can occur between minerals that share similar characteristics, such as valence shell electronic configuration, ionic radius, coordination number, geometric arrangement, and ligand exchanges (38). Both Fe and Mn have an affinity for binding to ferritin and transferrin. A high intake of one mineral may reduce the absorption and overall levels of the other mineral in the plasma, creating a counteractive interaction between these two elements (38).
5.2. Pumpkin Seed Oil Supplementation on the Serum, Liver, and Gastrocnemius Concentrations of TMEs
The role of TMEs in energy metabolism (5) and the increased excretion of Zn through sweat, urine, and redistribution among bodily fluids and tissues during physical exercise, along with an imbalanced diet, may highlight the importance of sufficient dietary intake of TMEs in physically active individuals (7, 32, 33). The results of this study indicated that the levels of Mg in the blood serum, as well as the levels of Zn, Mg, Manganese, and Fe in the gastrocnemius muscle, significantly increased after 6 weeks of pumpkin seed oil supplementation. These levels were comparable to those of the control group that received a saline solution (P < 0.05). We hypothesized that the transfer of essential trace elements to active organs like the gastrocnemius during physical activity had been compensated by the primary function of pumpkin seed oil in supporting the redox system's needs. Pumpkin, along with other functional foods and natural remedies, is being extensively studied for its therapeutic properties, as many pharmaceutical drugs can lead to several adverse effects (11). The pumpkin, a member of the Cucurbitaceae family, is considered a medicinal herb (11). Scientific evidence supports that pumpkin possesses various biological properties, such as anti-fatigue, antioxidant, and anti-inflammatory effects (11, 39). The health benefits of pumpkin seeds may be attributed to their composition of several macro- and micronutrients, including proteins, triterpenes, lignans, phytosterols, polyunsaturated fatty acids, antioxidative phenolic compounds, carotenoids, tocopherol, and minerals (11, 39, 40). The recommended dietary allowances (RDAs) for trace elements, including Zn, Mg, iron, manganese, and Cu, are as follows: 15 mg/day for Zn, 420 mg/day for Mg, 18 mg/day for Iron, 2.3 mg/day for manganese, and 3 mg/day for Cu, for adults (5, 41).
The current research found that pumpkin seed oil increased the levels of Zn, Mg, and Fe in the gastrocnemius tissue, while the levels of manganese and Cu remained unchanged. These findings suggest that pumpkin seed oil could serve as an ergogenic aid for athletes by replenishing TMEs and facilitating the elimination of waste products from physical activity. This study is the first to investigate the performance-enhancing effects of pumpkin seed oil on the levels of TMEs in the blood, liver, and gastrocnemius of healthy rats, confirming the potential of pumpkin seed oil supplementation in athletes. It addresses the gap between increased dietary Zn and Mg intake and the lower blood concentrations observed in previous studies (4, 39).
The research conducted by Skalny et al. explored the effects of Zn supplementation (15 mg/kg/day) on rats during a 10-minute treadmill exercise at a speed of 23 m/min. The study observed a significant increase in Zn levels in both the blood and cardiac tissue (36). While the study noted higher levels of Zn, excessive consumption of Zn can lead to negative effects. An excess of Zn in the body can increase the Zn/Cu ratio and result in the excretion of Cu from bodily fluids and tissues due to competition between Fe and Cu for receptor binding (5, 35). Moreover, empirical research has indicated that administering Zn and Fe simultaneously to rats with Fe deficiency is more effective in increasing blood Fe levels (40). The changes may occur due to Zn-induced modulation of Fe regulatory proteins (40). These findings are consistent with our results and support the observed simultaneous increase of Fe and Zn in the gastrocnemius muscle tissue. The beneficial role of Fe during exercise may be attributed to its ability to facilitate oxygen transport through its structural function in hemoglobin and myoglobin (38).
Unexpectedly, the addition of pumpkin seed oil did not affect Cu levels in rats subjected to exercise. This finding is in line with previous research suggesting antagonistic interactions between Zn and Cu, especially during absorption (42). Due to its redox activity, Cu may participate in Fenton-like reactions that generate reactive oxygen species (43). As a result, Zn supplementation may mitigate the oxidative stress and toxicity caused by Cu. It has been specifically shown that the relationship between the concentrations of Cu and Zn is directly related to the level of oxidative stress (44). According to our unpublished laboratory findings, the TME concentration in pumpkin seed extract exceeds that in pumpkin seed oil, suggesting that pumpkin seed extract may more effectively meet the body's TME demands during exercise and recovery.
The significant advantage of this research lies in the concurrent measurement of selected TMEs in three separate body samples, enabling us to make more reliable claims about the variations in TMEs caused by pumpkin seed oil supplementation. A limitation of the current study is the absence of an assessment of TME levels in urine, sweat, and erythrocytes, which could have enhanced the generalizability of the study's conclusions. This indicates that future research should examine the quantities of TMEs excreted in urine and sweat, as well as the efflux/influx between serum and erythrocytes.
5.3. Conclusions
The individuals in this study underwent six weeks of submaximal physical exercise, resulting in a noticeable downregulation of serum and gastrocnemius tissue levels of TMEs. Supplementing with pumpkin seed oil enhances the trace element resources in active muscle and may contribute to subsequent performance benefits. The findings of this study suggest that supplementing with pumpkin seed oil may serve as a beneficial nutritional approach to improve muscle performance. Additionally, the observed increases in TME resources within the same sample further support the idea that incorporating pumpkin seed oil supplementation into a nutritional regimen could substitute for chemical drugs for athletes during physical exercise.