1. Background
Stroke is a key contributor to patient mortality and is one of the main causes of disabilities globally (1, 2). In previous studies, it has been established that eye movement abnormalities, neurological deficits, arm and leg weakness, arm and leg paresis, gait ataxia, and subjective facial asymmetry are prominent indicators of post-stroke outcomes (3). Neurological assessment plays a crucial role in evaluating stroke patients and is a skill that has been practiced and refined by physicians for many years (4). Consequently, numerous post-stroke restorative modalities are under investigation, including brain stimulation, growth factors, monoclonal antibodies, cell-based therapies, drugs, robotic and telehealth devices, and activity-based therapies, all of which have shown benefits in neural repair and cognitive function remapping after stroke (5).
Exercise training is one of the stroke-dependent modalities with evidence suggesting its potential to modulate stroke-associated impairments, particularly through enhanced cerebral blood flow in infarct areas of the brain (6). Recovery of gait is a primary objective in neurological rehabilitation (7), and treadmill exercise is a widely recognized and well-studied training method in stroke recovery. Treadmill training has been shown to reduce post-stroke deficiencies (8). However, the effectiveness of neurological rehabilitation is strongly influenced by the intensity and duration of the training (9).
There is concern about the appropriate intensity and duration of treadmill exercise training, as higher intensity may lead to poorer outcomes following a stroke (10). While treadmill training is a standard rehabilitation practice, the optimal roles of intensity and duration in human studies remain unclear (8). Previous literature has underscored the importance of exercise intensity in stroke rehabilitation programs. Some studies suggest that gradually increasing exercise intensity may promote greater neurological recovery than low- or high-intensity regimens (11-13). Conversely, other studies indicate that high-intensity protocols result in superior neurological recovery compared to low-intensity protocols (12, 14). While low-intensity strategies may have a reduced effect on recovery, some evidence shows no significant differences in motor function recovery between low- and high-intensity training protocols (8).
In terms of duration, some studies report that shorter durations yield better neurological outcomes (14-16), while others find that longer durations are more beneficial (17-19). However, there is insufficient evidence regarding the effect of treadmill protocol intensity and duration on stroke-induced neurological deficits. The objective of this meta-analysis was to determine the effects of treadmill training on post-stroke neurological deficits in human studies. The findings may help guide physicians, healthcare professionals, and researchers in selecting the optimal intensity and duration of training protocols for stroke patients.
2. Evidence Acquisition
2.1. Definitions of Variables
In line with the criteria outlined by the World Health Organization (WHO), stroke is defined as the abrupt onset of clinical manifestations indicating focal (or global) dysfunction of cerebral function, lasting over 24 hours or leading to death, with no apparent cause other than of vascular origin (20). Additionally, a reduction of one or more points in the overall neurological scale score from entry to hospitalization was used as the criterion for defining neurological deficit (21).
In this meta-analysis, we included human neurological scales, such as assessments of eye movements, closed-eye balance, and the Modified Ashworth Scale (MAS). Therapeutic exercise or exercise therapy refers to a prescribed and supervised program in which patients perform repeated voluntary dynamic movements or static muscle contractions. These exercises may target specific body regions or involve the whole body, either with or without external resistance. The goal is to relieve symptoms, control inflammation (if present), improve range of motion, muscular endurance, strength, and power, and ultimately assist patients in returning to their daily and recreational activities through training (22). For the present investigation, we included the use of treadmills with body-weight support as part of the therapeutic exercise.
According to the American College of Sports Medicine (ACSM) and the American Heart Association (AHA), exercise-related adaptations for stroke survivors are expected to manifest after two to six weeks, with three sessions per week and approximately 20 - 60 minutes per session (23, 24). Therefore, in line with previous investigations, the duration of treadmill training was calculated based on the total minutes allocated for therapeutic exercise in each session, week, and training week(s). The training protocol was considered as high duration (more than 500 minutes) if the total training time exceeded 500 minutes; otherwise, it was categorized as low duration (less than 500 minutes) (8).
The AHA practical guidelines on exercise recommendations for stroke survivors suggest that intensity training should be performed at a level of 40% - 70% of peak oxygen uptake or heart rate reserve, 50% - 80% of maximal heart rate, or a perceived exertion level of 11 - 14 on the 15-category Rated Perceived Exertion (RPE) scale (24). However, due to challenges in practical application, several studies have demonstrated that a training intensity of 2 km/h (or 0.6 m/s) is more feasible for stroke patients (8, 25, 26). In line with previous studies, we defined treadmill training intensity as the speed of treadmill training during exercise therapy. Training intensity was categorized as high intensity (more than 0.6 m/s) if the intensity exceeded 0.6 m/s, otherwise it was classified as low intensity (less than 0.6 m/s) (8). Training difference refers to the difference in intensity and duration between the experimental group and the control group, or the difference compared to pre-test values (8, 27).
2.2. Selection Process of Relevant Studies
We selected appropriate stroke-related articles for inclusion based on predefined criteria, such as study design, training intensity, duration conditions, and outcome measures, from January 1980 to March 2020 using the PubMed search engine. Headlines and keywords related to medical topics were sought out from different platforms (8). Our previous data pool, comprising studies up to March 2020, was further updated (Box 1).
| Details |
|---|
| ("exercise"[MeSH Terms] OR "exercise"[All Fields]) AND ("stroke"[MeSH Terms] OR "stroke"[All Fields])*("stroke"[MeSH Terms] OR "stroke"[All Fields]) AND ("cerebrum"[MeSH Terms] OR "cerebrum"[All Fields] OR "cerebral"[All Fields] OR "brain"[MeSH Terms] OR "brain"[All Fields])) AND ("exercise"[MeSH Terms] OR "exercise"[All Fields])*(stroke[Title] AND cerebral[Title]) AND exercise[Title]*(stroke[Abstract] AND cerebral[Abstract]) AND exercise[Abstract]*(stroke[Abstract] AND exercise[Abstract]) AND cerebral[Abstract]) AND brain[Abstract]) AND physical[Abstract]) AND training[Abstract])*Post-ischemic[All Fields] AND ("exercise"[MeSH Terms] OR "exercise"[All Fields]* ("exercise"[MeSH Terms] OR "exercise"[All Fields]) AND ("physical examination"[MeSH Terms] OR ("physical"[All Fields] AND "examination"[All Fields]) OR "physical examination"[All Fields] OR "physical"[All Fields])) AND ("cerebrum"[MeSH Terms] OR "cerebrum"[All Fields] OR "cerebral"[All Fields] OR "brain"[MeSH Terms] OR "brain"[All Fields])) AND ("stroke"[MeSH Terms] OR "stroke"[All Fields])) AND ("brain"[MeSH Terms] OR "brain"[All Fields])*(post-exercise[All Fields] AND ("cerebrum"[MeSH Terms] OR "cerebrum"[All Fields] OR "cerebral"[All Fields] OR "brain"[MeSH Terms] OR "brain"[All Fields])) AND ("ischaemia"[All Fields] OR "ischemia"[MeSH Terms] OR "ischemia"[All Fields])*Hemiparetic[All Fields] AND treadmill[All Fields] AND ("1980/01/01"[PDAT] : "2018/03/01"[PDAT]). |
2.3. Research Entry Criteria
From our search results, we identified 87 studies that were potentially suitable, with six meeting our inclusion criteria (Table 1). Among these, four studies involved human participants. We also examined studies that included one or more independent variables (such as intensity and/or duration of treadmill training) and used a neurological scale as the dependent variable, as well as studies that featured multiple independent and dependent variables. Additionally, the amount of body-weight support (BWS) used during treadmill training (10% to 40% of body mass) was considered a crucial variable in the studies.
Abbreviations: N/A, not applicable; BWS, body-weight support.
a The meta-analysis biases were consisted of training intensity, duration, protocol expression, safety (heart rate monitoring or BWS), overload, and neurological scale expression, respectively.
Our analysis focused exclusively on studies that clearly explained the training intensity using measurements in meters per minute (m/min), centimeters per second (cm/s), meters per second (m/s), or kilometers per hour (km/h), all of which were standardized to m/s for human experiments. The training duration was determined by calculating the minutes per session, the number of sessions per week, and the total number of training weeks, which were then converted into total minutes (30). Neurological recovery following a stroke was assessed using standard neurological assessment scales, though the specific names of these scales were not disclosed by the authors (Table 1) (30, 31).
2.4. Confounding Factors
In our study, potential confounding factors included the age difference at the time of stroke as well as the functional status prior to the occurrence of stroke. Additionally, the amount of BWS used during treadmill training (10% to 40% of body mass) was considered another confounding factor.
2.5. Research Biases
Similar to other meta-analyses, the present study was subject to several potential biases, such as training intensity, training duration (≥ two weeks), clarity of the training protocol, training safety (e.g., heart rate monitoring or BWS), training overload, and expression of neurological scales. Studies that addressed or covered at least half of the above-mentioned biases were included in the data pooling (32, 33).
2.6. Research Data Extraction
The author succinctly outlined the findings of human studies, including key details such as: (1) the first author's name and publication year; (2) applied data for every study, such as the number of subjects, sessions, days, and weeks of training; (3) a description of treatment specifics, including the intensity and duration of treadmill training during the entire therapeutic exercise session, which were then converted into meters per second (or per minute) and minutes, respectively; (4) classification of the therapeutic protocols into categories such as: (a) low intensity; (b) low duration, low intensity; (c) high duration, high intensity; (d) low duration, and high intensity - high duration, based on studies conducted on humans (34-37); (5) presentation of the study findings as mean ± standard deviation (SD).
When data was unavailable for pooling, efforts were made to calculate the necessary information or to contact the authors for additional data; otherwise, studies were excluded due to lack of accessible data. Additionally, we contacted the corresponding authors of conference abstracts for full-paper publications, where possible, and consulted professionals in the relevant field for further insights.
2.7. Classification of Research Studies
Before commencing therapeutic exercise, a comprehensive appraisal is necessary to assess neurological deficits in human studies (7). In this meta-analysis, we predominantly employed neurological scales for subgroup categorizations. The neurological scales included assessments of eye movements, closed-eye balance, and the MAS for human studies. Subgroup classifications for human studies were determined based on previous research and are as follows (8, 34-37):
(1) Low intensity (0.6 m/s or less) - low duration (500 minutes or less) training protocol
(2) Low intensity (0.6 m/s or less) - high duration (more than 500 minutes) training protocol
(3) High intensity (more than 0.6 m/s) - low duration (500 minutes or less) training protocol
(4) High intensity (more than 0.6 m/s) - high duration (more than 500 minutes) training protocol
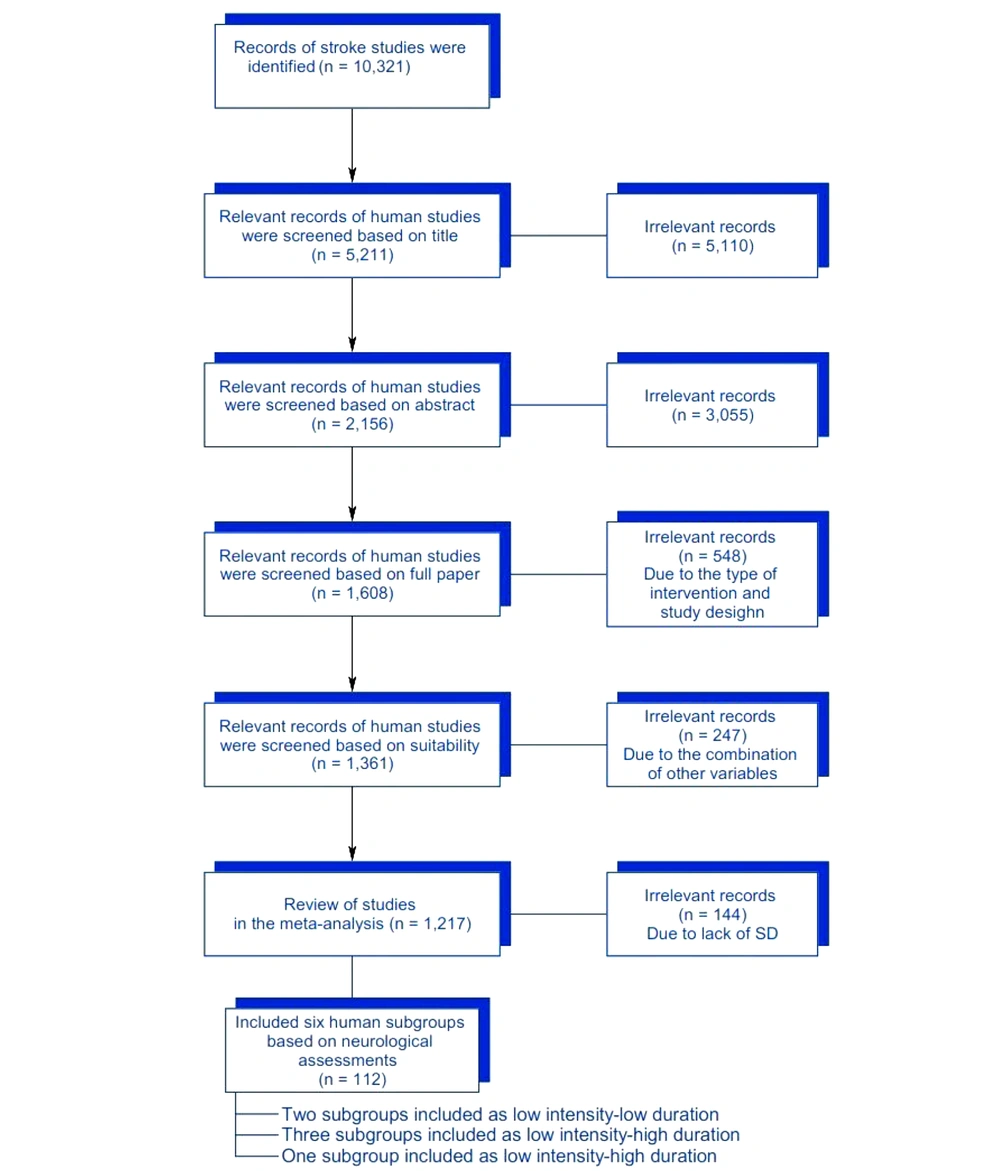
According to the neurological scales, we included four out of 28 studies in this meta-analysis. The subgroups of human studies included two subgroups categorized as low intensity-low duration protocol (14, 28), with one subgroup being excluded. Three subgroups were classified as low intensity-high duration protocol (14, 17, 29), and one subgroup was categorized as high intensity-high duration protocol (14). Notably, there was no study or subgroup on humans that was categorized as high intensity-low duration training protocol (Figure 1).
2.8. Statistical Analysis
Quantitative data were recorded as the mean ± SD following the specified criteria. Continuous data were then processed using the "Metan" command (38, 39) to calculate the mean, SD, and sample size for each group. Standard mean differences (SMD) with 95% confidence intervals (CI) and weight percentages for each study or subgroup were subsequently assessed using the "Metan" command, as described in prior research (8).
Egger's funnel plot and Egger's test, using the "Metabias" command, were applied to detect publication bias, following the method outlined by Egger et al. (40). The I-squared statistic (I2) was used to evaluate the level of heterogeneity, based on Higgins et al.'s recommendations (41). To account for variability among multiple studies, a random effects model was preferred over a fixed effects model (42).
All statistical analyses were performed using Stata 12 (StataCorp, USA), and study figures were generated using GraphPad Prism 6.01.
3. Results
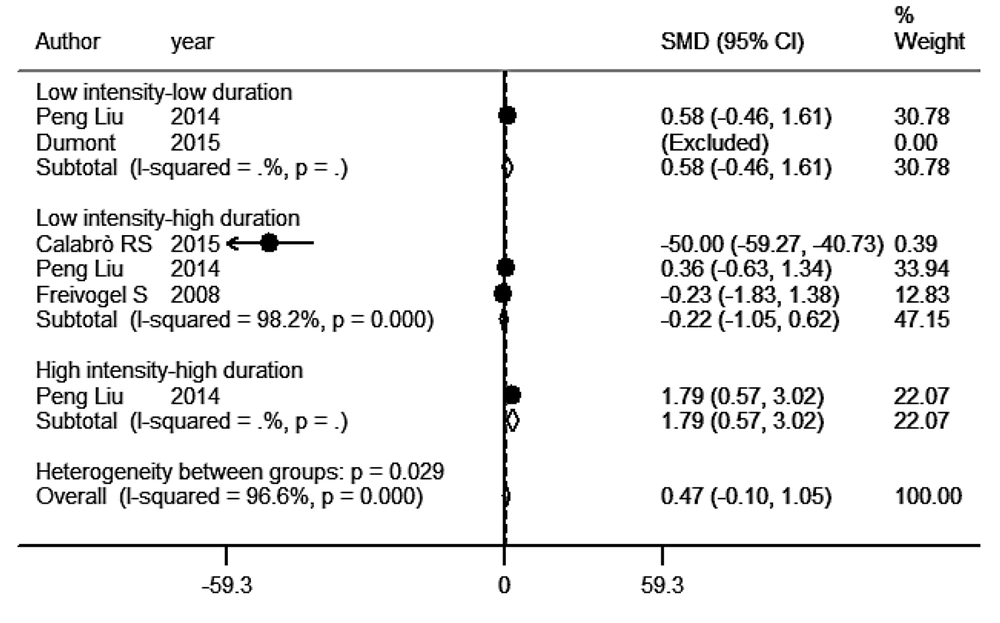
Figure 2 illustrates the SMD with 95% CI for neurological rehabilitation in the dominant training protocol categories. Four studies (N = 112) examined the use of a motorized treadmill for walking, which included patients undergoing treadmill rehabilitation protocols (14, 17, 28, 29). One study (28) did not have a clear post-stroke rehabilitation SMD. The CIs for the SMD of both low intensity-low duration subgroups and high intensity-high duration subgroups were greater than zero. However, the CI for the SMD of the low intensity-high duration subgroups was significantly less than zero. Additionally, one subgroup from the low intensity-low duration protocol was excluded by Stata from the meta-analysis due to zero weight.
Recovery of neurological deficit following the treadmill training strategies in stroke patients. In this meta-analysis, we did not see a significant reduction in the neurological deficit after the treadmill training interventions, but there was nonsignificant decline in the neurological deficit following the low intensity-high duration and low intensity-low duration protocols (P > 0.05). However, the high intensity-high duration intervention significantly decreased the neurological deficit in stroke patients (P = 0.0001). Altogether, the current meta-analysis displayed that, regardless of training intensity and duration, treadmill training did not have a significant effect on recovery of the neurological deficit (P > 0.05).
The six human studies included in the analysis exhibited a high level of heterogeneity (I2 = 96.6%; P < 0.0001). While a negative SMD was observed, both the low intensity-low duration and low intensity-high duration protocols resulted in a reduction in neurological deficit, though the difference was not statistically significant (P = 0.277 and P = 0.615, respectively). Conversely, a positive SMD was observed in the high intensity-high duration protocol, indicating an increase in neurological deficit with higher intensity (SMD = 1.792; 95% CI = 0.566 to 3.02; P = 0.004). Nevertheless, there were no significant differences in SMD across the various subgroups in the study (P = 0.109; Figure 2). Furthermore, the Egger's funnel plot and test with a 95% confidence interval indicated no publication biases in any of the study subgroups (t = 2.09, P = 0.104).
4. Discussion
Regarding recent literature, prior research has increasingly demonstrated that exercise training is being recommended as a critical component of stroke rehabilitation programs, given the growing body of evidence supporting its benefits in mitigating neurological and functional impairments following stroke (43). The outcomes of our earlier study strongly suggest that treadmill training, regardless of intensity or duration, results in significantly better motor function rehabilitation compared to the absence of training (8). However, the specific impact of exercise training intensity and duration on neurological recovery in stroke patients remains unclear.
To address this, we evaluated whether training duration, intensity, or a combination of both influenced the primary neurological outcomes in stroke-related human studies. The current meta-analysis revealed that approximately 79.4% of the combined studies did not provide precise details on treadmill training protocols, thus failing to adequately address biases in the analysis. In contrast, only 20.6% of the studies accounted for all six biases considered in the meta-analysis. Furthermore, the meta-analysis highlighted a significant decline in the number of stroke-related treadmill training investigations over the last three years.
Our research findings indicate that 2 out of 7 interventions (28%) provided significant evidence of beneficial effects on neurological and functional recovery, based on the results of our meta-analyses.
A significant number of stroke-related training studies indicate that treadmill training yields comparable results to conventional therapy, suggesting that similar outcomes can be achieved with the control intervention, without conflicting findings. It is important to note that while adverse SMDs in neurological deficit were observed in the low intensity-low duration and low intensity-high duration protocols, these differences were not statistically significant. Only one study (29) reported a negative SMD, indicating that the low intensity-high duration protocol had a positive impact on neurological deficit. Despite this, there was no significant difference in the overall SMD across the subgroups. On the other hand, the high intensity-high duration protocol showed a positive SMD, suggesting that as treadmill intensity increases, neurological deficit tends to worsen.
Furthermore, it has been shown that intensity plays a key role in inducing stress. Intense training leads to elevated stress levels, which are associated with reduced hippocampal brain-derived neurotrophic factor (BDNF) levels, a marker of neural activity (2). Previous studies have demonstrated that exercise training can enhance neurological recovery by boosting neural progenitor cell levels through the activation of the IGF-1/Akt and BDNF signaling pathways (2, 44, 45). Therefore, practitioners opting for the high intensity-high duration protocol should consistently monitor neurological outcomes that may be negatively impacted by the protocol.
Despite the findings, this meta-analysis indicated a lack of statistical power or summary effect sizes (SESs) for all neurological outcomes (SESs = 0.496), emphasizing the need for additional human studies. While a notably negative SMD was not detected in low intensity training protocols, these methods still showed positive impacts on motor functional recovery. Although the meta-analysis was carried out meticulously, it faced methodological limitations such as language restrictions and missing data (33, 46, 47). Additionally, the assessment of subgroups or sub-studies is limited by the observational nature of the included research, and caution is advised in interpreting these findings until stronger evidence is available (33, 39-41, 46, 47). Controlled variables differed between studies, and future human studies should further assess neurological outcomes following treadmill training.
Given the limited number of subgroups (six) that examined neurological recovery after treadmill training, further studies are needed to validate and expand upon these findings.
4.1. Conclusions
In conclusion, our findings deliver several key messages. First, this meta-analysis includes studies with the least biases, strengthening the reliability of the results. Furthermore, it confirms the significant impact of treadmill training protocols in enhancing neurological recovery and reducing the risk of disability in stroke patients. Additionally, the current meta-analysis suggests that practitioners using the high intensity-high duration protocol should regularly monitor neurological outcomes, as this protocol may negatively impact recovery in some cases. Lastly, to make it easier to achieve the functional and neurological improvements observed in these meta-analyses through regular exercise, it is essential to specifically identify the training intensity and duration that show strong evidence of benefiting stroke patients. Furthermore, determining which type of study provides valid evidence for this is crucial for optimizing stroke rehabilitation protocols.