1. Background
The lung is a biological system with a biotensegrity structure, stabilized from the tissue to the sub-cellular level. Sensor cells detect mechanical stimuli, regulating genotype and cell function. Effector cells generate or enhance chemical signals in response. However, pathological conditions like Chronic Obstructive Pulmonary Disease (COPD) can cause alterations in the matrix's composition, leading to mechanical transmission issues that affect lung cell integrity (1, 2).
Chronic Obstructive Pulmonary Disease, the sixth most common cause of disability in developed countries, is 95% related to smoking (3). Inhaled cigarettes cause inflammation of the alveoli, leading to airflow restriction. This can be irreversible due to changes in lung architecture, including pulmonary emphysema. This results in the destruction of elastin tension fibers, a key protein in the lungs responsible for their elastic properties. Natural regeneration of elastin is minimal in healthy individuals, with a half-life of 15 years, but it increases during lung diseases (4).
Elastin resistance is crucial for its lifespan, and cigarette smoke-induced emphysema in rats was caused by the removal of the matrix metalloproteinase-12 (MMP12) gene. This gene is often associated with lung disease and elastin degeneration (5). Antiproteases are used to treat emphysema, but there is evidence of an autoimmune reaction towards transforming growth factor-beta 1 (TGF-β1), which regulates elastic fiber density in alveoli. TGF-β1 upregulation is linked to emphysema development, but it also protects against tissue damage and suppresses disease progression. Chronic obstructive pulmonary disease can cause gas exchange disruption, bronchoconstriction, pulmonary edema, muscle fiber changes, and decreased metabolic and physical function. Targeted exercise interventions to control sensitivity and cellular-tissue repair, especially in the lungs, are fundamental strategies for managing COPD (6). Despite this, the impact of different movement patterns on the behavior of tensile stress elements at various stages along mechanical intracellular signaling pathways remains unknown.
Light exercise training can improve aortic wall repair in hypertension-prone rats and increase elastin content in healthy rats (7). However, in rats exposed to cigarette smoke, intermittent aerobic training reduced vascular tissue damage without significant changes in lamina and elastin levels. Aerobic exercise on a moderate-intensity treadmill did not significantly alter the resistance and dynamic elasticity of the respiratory system. Elastin protein, a marker of lung cell tension, undergoes minimal synthesis after birth, making it crucial to protect its integrity and well-being (8).
Research shows that nerve stimulation can decrease MMP12 and inflammatory cytokines in rats with COPD (8). Aerobic exercise can also reduce MMP12 levels induced by chronic allergic airway inflammation (9). After 24 weeks of aerobic training, rats exposed to cigarette smoke showed a decrease in MMP9 and MMP2 activity. However, Vieira Ramos et al. (2018) did not observe ECM remodeling in COPD patients after exercise (5). Resistance training for five to 12 weeks increased MMP9 and MMP2 in both humans and animals, while acute resistance activity may decrease these variables. Aerobic exercises yielded contradictory results (10).
Stress-induced changes in COPD patients are linked to increased expression of fibroblast stem cells and activation of the TGF-β1 signaling pathway in the extracellular matrix. This is due to mechanical membrane sensors mediated through integrin in cell survival (11, 12). Studies have shown that exercise can prevent the progression of emphysema, hyperplasia of mucus cells, and recurrence of pulmonary fibrosis in mice (12). Moderate-intensity aerobic exercise can reduce the production of oxidative stress and inflammatory factors, as well as TGF-β1, leading to a decrease in elastic tissue regeneration in individuals with asthma. Additionally, it has been found that TGF-β1, a cytokine involved in tissue regeneration, increases following intense and continuous interval training in certain studies. Muscle damage caused by eccentric activity can result in increased expression of MMP9, TIMP1, and TGF-β1, leading to scar tissue formation and delayed recovery (13). Therefore, the optimal effectiveness of exercises depends on the correct selection of sequence, intensity, duration, and type of exercise. Interval training with appropriate intensity may provide more favorable conditions for cell repair and serve as an effective treatment strategy (6, 13).
2. Objectives
The present study operates under the premise that different elements of the extracellular matrix (ECM) are essential for preserving the lung's optimal biotensegrity. Cigarette smoke negatively impacts the TGF-β1, MMP12 signaling system, and elastin fiber flexibility. Furthermore, preserving the elasticity of lung tissue is crucial for managing COPD. However, there has been limited research on the impact of exercise on molecular cells in this context. Therefore, the current study investigated the effects of six weeks of aerobic interval training (AIT) on the behavior of variables related to elastin, MMP12, and TGF-β1 in CS-COPD rats.
3. Methods
3.1. Animals and Experimental Design
Male Wistar rats weighing between 180 and 220 g (eight weeks old) and reared in the animal facility at Baqiyatallah University of Medical Sciences in Tehran, Iran, were utilized in the experiments, following the NIH Guidelines for Animal Research (ethical code: IR.BMSU.REC.1400.117). The animals were provided unrestricted access to both food and water, residing in regulated settings featuring a 12-hour alternating light-dark cycle at a temperature of 22°C. The standard diet supplied contained 23% protein, 4% fat, 42% carbohydrates, 1% calcium, 0.25% tryptophan, 0.33% methionine, 1.15% lysine, 0.7% threonine, 0.65% phosphorus, 0.5% salt, 4% fiber, and an energy content of 2900 kcal/kg. A total of four groups, each comprising 28 male Wistar rats, were randomly allocated:
(1) The control group was given 150 µL of normal saline intraperitoneally.
(2) The Exe group engaged in aerobic interval training on a rodent treadmill for five days per week over six weeks. The training consisted of 7 sets of intervals, each consisting of 4 minutes at 80 - 90% VO2max, followed by 3 minutes at 65 - 75% VO2max.
(3) The experimental group, designated as CSE, was subjected to a weekly intraperitoneal injection of 150 µL of CSE for six weeks.
(4) The CSE + Exe group received both CSE and performed Exe, following the same protocol as groups 3 and 2.
3.2. Cigarette Smoke Extract
The CSE used in the current study was prepared based on previous research (14, 15). To create the CSE-PBS solution, three Winston cigarettes were burned, and the resulting smoke was transferred to a container containing 10 ml of PBS using a vacuum pump. The CSE-PBS solution was made fresh for each set of experiments and then filtered through a 0.22 µm Millipore filter to remove bacteria and particles. The rats in the healthy control group were injected with 150 µL of normal saline intraperitoneally. On days 7, 14, 21, 28, 35, and 42, the CSE groups were injected with 150 µL of the CSE-PBS solution intraperitoneally (14, 15).
3.3. Aerobic Interval Training
To familiarize the rodents with treadmill locomotion prior to commencing the main training regimen, they participated in a five-minute session at a velocity ranging from 8 to 10 meters per minute, without any incline, over five sessions spanning one week. The aerobic interval training (AIT or Exe) regimen involved a ten-minute warm-up at 50 - 55% of maximal oxygen uptake (VO2max), followed by seven sets of interval exercises. These exercises consisted of four minutes of aerobic activity at 80-90% VO2max, followed by a three-minute rest period at 65 - 75% VO2max. The training session concluded with a five-minute cool-down session. A test for the indirect measurement of VO2max was carried out on Wistar rats every two weeks to calibrate running pace and maintain training intensity.
3.4. Gene Expression
Using Qiazol (Qiazol lysis reagent, USA), total RNA was extracted from lung tissue in a sterile, RNase-free tube. The RNA's purity and concentration were assessed by measuring the absorbance at 260 and 280 nm (the A260/280 ratio) using a NanoDrop ND-100 spectrophotometer (Thermo Scientific, USA). The RevertAid cDNA synthesis kit (Fermentas, Germany) was used to convert RNA into cDNA in a 25-μL volume, following the manufacturer's instructions. The polymerase chain reaction (PCR) amplification reaction consisted of 2 μL of the cDNA synthesis reaction, 12.5 μL AccuPrime SuperMix I (Fermentas, Germany), 10.1 μL of distilled water, and 0.2 μL of each forward and reverse primer (100 μmol/L). The NCBI BLAST Tool and Primer3 software were used to design and validate the primers.
In real-time PCR, 500 ng of the newly produced cDNA was used to measure relative gene expression. The PCR reactions were conducted using 12.5 μL of SYBR Green Premix 2× (Takara, Japan) and 25 μL of mixed primers (10 p-molar). The thermal cycling protocol included an initial step at 95°C for 10 s, followed by 40 denaturation cycles at 94°C for 5 s, and annealing and extension at 60°C for 34 s. The ΔΔCT method was employed to quantify the relative expressions of lung elastin, MMP12, and TGFB1 genes. The Ct samples were compared to those of the internal control (GAPDH). Real-time PCR was performed using the ABI detection system from Applied Biosystems, USA. Each reaction was repeated five times. The specificity of the PCR reaction was verified through electrophoresis and melting curve analysis. Gene analysis was conducted using GraphPad Prism 5. The primer sequences used are listed in Table 1 (14, 15).
| Gene | Primer Sequence |
|---|---|
| Elastin | |
| Forward | GGTGCTTCTTCTTTGTGGGG |
| Reverse | GAGGCTTCTGGTACTTGGGT |
| MMP12 | |
| Forward | GAGTCCAGCCACCAACATTAC |
| Reverse | GCGAAGTGGATCAAAGACAG |
| TGFB1 | |
| Forward | TACGCCAAAGAAGTCACCCG |
| Reverse | GTGAGCACTGAAGCGAAAGC |
| GAPDH | |
| Forward | GCATCTTCTTGTGCAGTGCC |
| Reverse | GATGGTGATGGGTTTCCCGT |
3.5. Hematoxylin and Eosin (H&E) Analysis
H Hematoxylin and Eosin E analysis was used to examine lung tissue damage caused by CSE. A fragment of pulmonary tissue was immersed in a 10% formalin solution for 24 hours to ensure fixation. Standard H&E staining techniques were then applied, allowing for the visual inspection of the morphological characteristics of the affected area. This assessment utilized a state-of-the-art DP73 digital microscope after embedding the tissue in paraffin, sectioning, and performing H&E staining (14, 15).
3.6. Statistical Analysis
The data were presented as the mean value along with the standard deviation. The Kolmogorov-Smirnov test was employed to validate the normality of the distribution. Following this, statistical significance was evaluated through a one-way analysis of variance (ANOVA), followed by detailed exploration using the Tukey post hoc test (via GraphPad Prism version 9.01). The level of significance was set at an alpha of 0.05.
4. Results
4.1. Histology
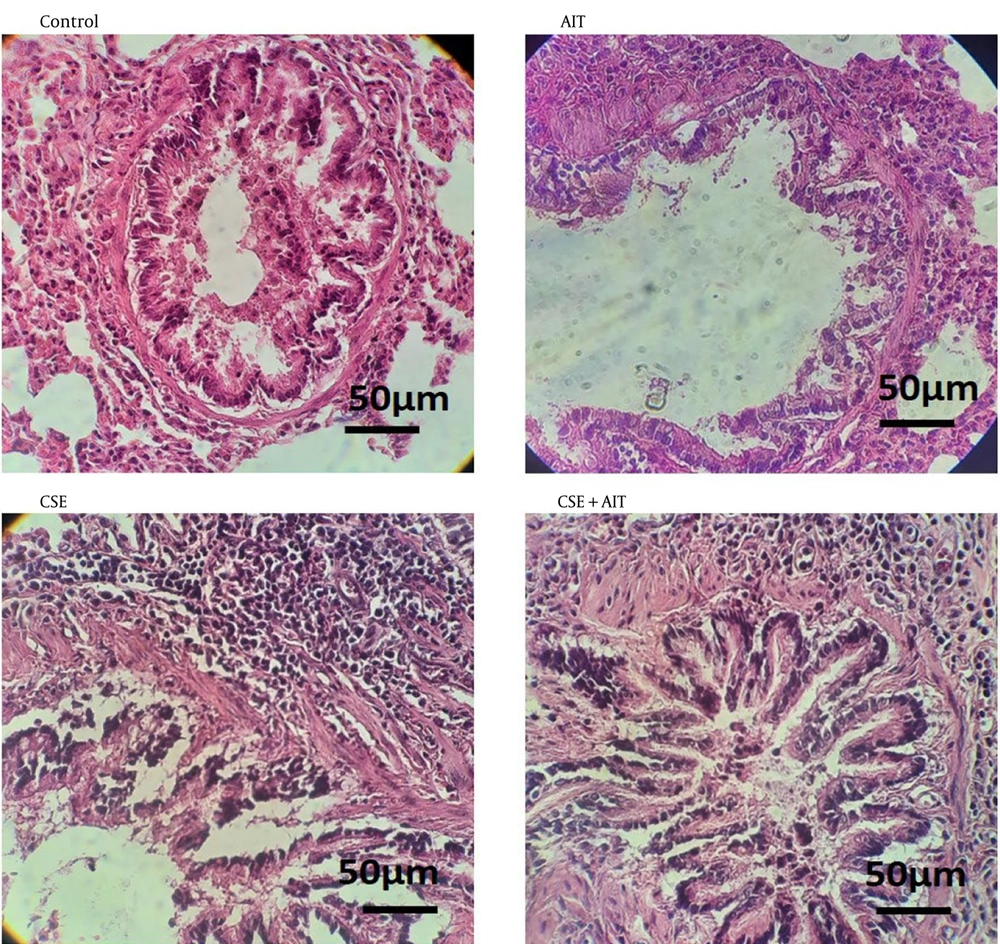
The qualitative findings regarding pulmonary histological changes are presented in Figure 1. Upon examination of the images, it appears that the healthy control group shows better tissue cohesion. Conversely, the CSE group displays signs of inflammation in the lung parenchyma, with an infiltration of inflammatory cells. Nodules can be observed in some areas of the lung parenchyma, particularly around the bronchioles and alveoli. Additionally, the epithelial cells of the bronchioles appear apoptotic, indicating damage to the sublayer of the isolated and muscular mucosa. Furthermore, damaged mucous cells are shedding into the bronchial duct. The study found that the cigarette and exercise groups experienced high rates of tissue damage, while the CSE + AIT group showed lower damage levels and improved tissue cohesion.
4.2. Gene Expression
4.2.1. Elastin
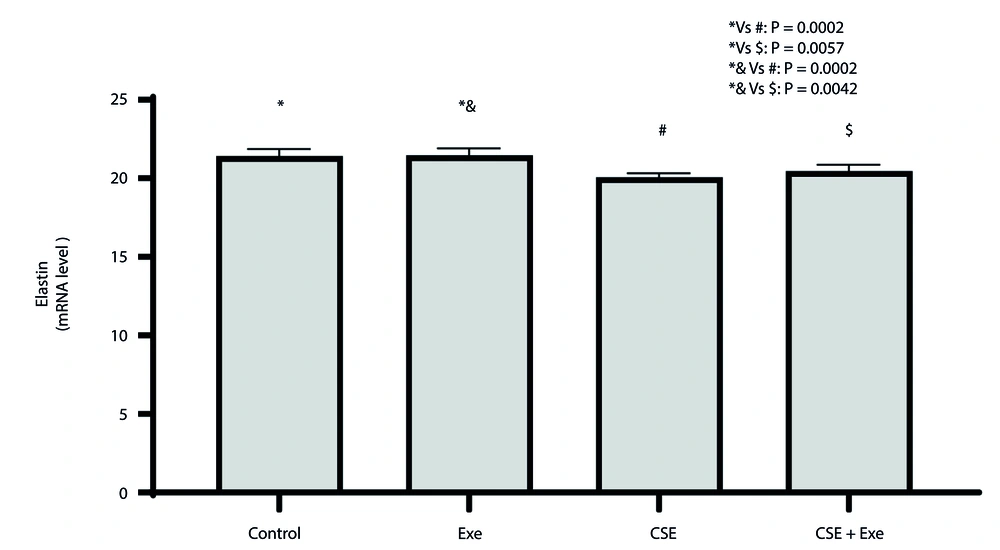
The changes in elastin expression in lung tissue are depicted in Figure 2. A significant difference was observed between the various research groups based on the results of the ANOVA test (P = 0.0001, F = 16.40). The Tukey test revealed a significant decrease in elastin gene expression in lung tissue in the CSE and CSE + Exe groups compared to the healthy control group (P = 0.0002 and P = 0.0057, respectively). Furthermore, the CSE and CSE + Exe groups showed a significant decrease in elastin gene expression in lung tissue compared to the Exe group (P = 0.0002 and P = 0.0042, respectively).
4.2.2. Matrix Metalloproteinase-12
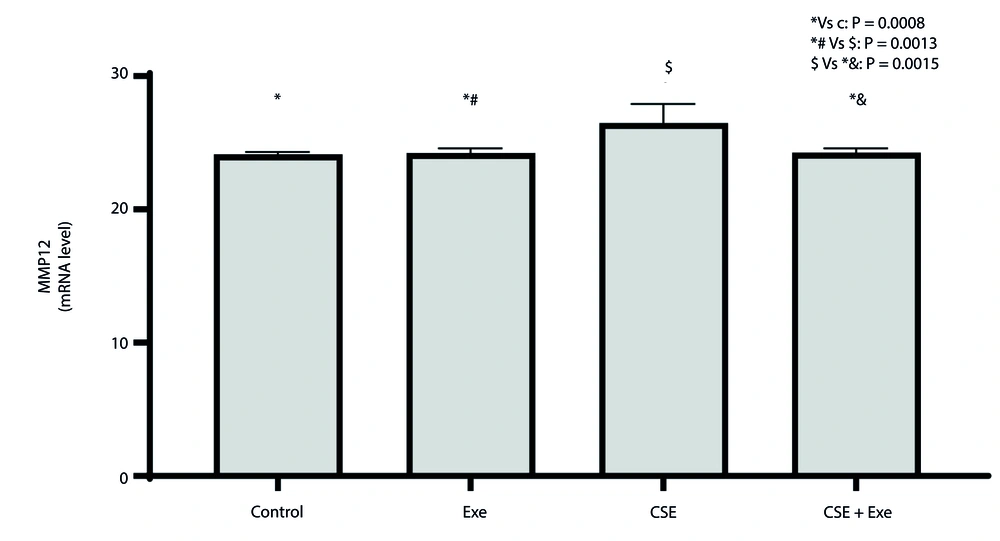
The changes in MMP12 expression in lung tissue are depicted in Figure 3. A significant difference was observed between the various research groups based on the results of the ANOVA test (P = 0.0003, F = 11.29). The Tukey test results showed that the CSE group had a significant increase in lung MMP12 gene expression compared to the healthy control and Exe groups (P = 0.0008 and P = 0.0013, respectively). On the other hand, the CSE + Exe group showed a significant decrease in MMP12 gene expression in lung tissue compared to the CSE group (P = 0.0015).
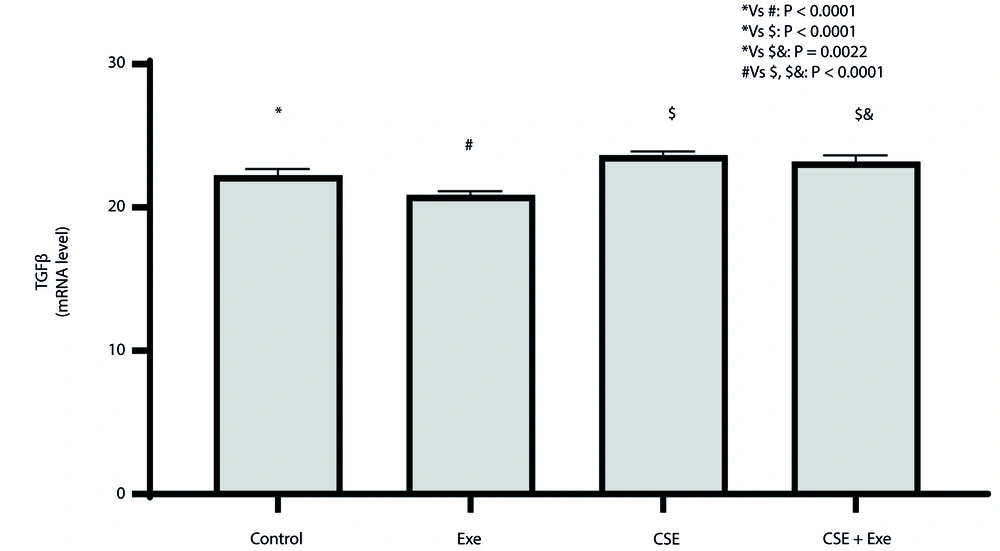
4.2.3. TGF-β1
The changes in TGF-β1 expression in lung tissue are depicted in Figure 4. The results of the ANOVA test reveal a significant difference among the various research groups (P = 0.0001, F = 64.69). Further analysis using the Tukey test demonstrates that the Exe group exhibits a significant decrease in TGF-β1 gene expression compared to the healthy control group (P < 0.0001). Both the CSE and CSE + Exe groups showed a significant increase in TGF-β1 gene expression in lung tissue compared to the healthy control group (P < 0.0001 and P = 0.0022, respectively). Additionally, the CSE and CSE + Exe groups exhibit a significant increase in TGF-β1 gene expression in lung tissue relative to the Exe group (P < 0.0001 for both).
5. Discussion
In the current study, we examined the impact of a six-week aerobic interval training regimen on elastin behavior by tracking alterations in MMP12 and TGF-β1 levels in rats exposed to cigarette smoke. Maintaining the integrity of elastin is crucial for the uniform transmission of mechanical forces throughout the lung, which is essential for its healthy function (16). However, direct exposure to cigarette smoke is a well-documented underlying cause of COPD, highlighting the significant role of the extracellular matrix in this condition (17).
Our findings revealed a significant decrease in elastin levels (P = 0.0002) in the group exposed to cigarette smoke compared to both the control and exercise groups. Notably, the CSE + Exe group exhibited a smaller and statistically insignificant decrease in elastin levels compared to the CSE group after six weeks of intermittent aerobic exercise. Exposure to cigarette smoke and exercise activates different signaling pathways in various tissues, resulting in changes in elastin content and tissue biotensegrity (18, 19).
In line with the findings of the current study, it was reported that smoke-exposed rats exhibited increased arterial elastin fractures, leading to heightened tissue stiffness. Although six weeks of moderate-intensity aerobic exercise significantly reduced tissue damage and increased nitric oxide (NO) levels, there were no significant changes in reversing the effect of elastin fractures (4). According to the potential mechanisms of matrix degradation and the development of emphysema due to cigarette smoke, various compounds are released from structural cells and alveolar macrophages, leading to an influx of macrophages, lymphocytes, and neutrophils (17).
A 6-week endurance exercise study in healthy rats revealed increased elastin content in skeletal muscle and the heart but not in the aorta, suggesting improved enzyme adaptability and conditioning responsible for ECM regeneration (7). However, the increase in elastin levels in the training group during the current exercise regimen was not statistically significant. Elastic filaments are particularly challenging to repair due to their small size, molecular complexity, and the requirement for multiple auxiliary proteins to facilitate fiber assembly and preserve tissue tensegrity (16).
Flo et al. (2006) did not observe a significant change in resistance and dynamic respiratory elasticity when assuming a negative effect of increased mechanical stress on degraded tissue caused by papain modeling emphysema during aerobic exercise on a moderate-intensity treadmill (20). Heightened tension and intense mechanical stress during exercise may be linked to higher respiratory rates, leading to more significant deformation of collagen and elastin fibers in the affected areas and exacerbating emphysema conditions (20, 21). Exercise, particularly in the early stages of a disease, can enhance positive tissue adaptation and prevent tissue deterioration. However, the type of exercise method, whether systemic or isolated, or the application of direct nerve stimulation to healthy or damaged tissue, has varying effects (8).
Chronic Obstructive Pulmonary Disease is a polygenic disease with four critical pathways: Inflammatory mediators, protease-antiprotease balance, xenobiotic and antioxidant metabolizing enzymes, and proteins involved in hyperactive airways (22). Cigarette smoke triggers pro-inflammatory reactions in lung cells, releasing cytokines like IL-1, IL-6, IL-8, IL-17, IL-18, IL-32, and TNF, and activating excessive amounts of elastases (8). Matrix metalloproteinase-12 is a macrophage-derived elastase responsible for smoking-related emphysematous changes. It activates pro-TNF to become active TNF, thereby enhancing the inflammatory process. Elevated MMP12 expression in the lower airways can directly damage the alveolar wall, resulting in matrix degradation and the release of elastin fragments. As a result, by creating a positive feedback loop, it appears to increase macrophage activity further (23). The lung is an organ that contains numerous macrophages, which play a central role in various processes, from inflammation to fibrosis, in close association with cytokines (8). This process involves an innate immune response to smoke, a protease feedback reinforcement system driven by matrix degradation, and elastin breakdown (18).
In line with previous reports, MMP12 levels in the CSE group of the current study increased significantly compared to other groups. However, after six weeks of periodic exercise, a significant decrease in MMP12 was observed in the CSE + Exe group. To date, no study has observed the impact of periodic aerobic exercise on changes in MMP12 gene expression in the epithelial lung tissue of the COPD-CS model. In a study on the impact of 12 weeks of nerve stimulation on the gastrocnemius muscle of rats with COPD, a decrease in MMP12 levels and inflammatory cytokines was observed, though no change was noted in the morphological alveolar space (8). The protective role of aerobic exercise has been reported to reduce MMP12 levels caused by high inflammation in chronic allergic airways (9). A review study citing specific findings revealed that the MMP response to exercise was more closely associated with the type and duration of exercise in various models. The study suggests that resistance training for five to 12 weeks can increase MMP9 and MMP2 levels in both human and animal models. However, acute resistance activity may lead to a reduction in these variables. Aerobic exercise also yielded conflicting results regarding these two variables (10). MMPs are also regulated by TGF-β1, an essential mediator of inflammatory responses (17).
The study revealed a significant rise in TGF-β1 levels in the CSE group compared to the healthy control and exercise control groups. The uncontrolled rise in TGF-β1 levels in COPD patients indicates the severity of the disease (24). TNF-α, released by MMP12, activates neutrophils and macrophages in the lung, which may influence the activation and secretion of TGF-β1. Studies have suggested a connection between TGF-β1 and the balance of MMP/TIMP in pathological conditions. The active TGF-β1 signaling pathway and increased myofibroblasts are likely to mediate the remodeling of fibrogenic airways, which are impaired in mice with MMP12 defects (25). On the other hand, moderate-intensity aerobic exercise reduces the production of oxidative stress and inflammatory factors. At the same time, TGF-β1 contributes to a decrease in the remodeling of elastic tissues in individuals with asthma (11). The effect of exercise on the modulation of the TGF-β1 signaling pathway has been shown to reduce inflammation and oxidative stress (11).
The study reveals that regular exercise can decrease TGF-β1 levels, suggesting it may affect inflammatory and oxidative pathways. In a study on inflammatory responses and exercise-modulated epigenetic markers after 24 training sessions under reduced inflammatory conditions, a decrease in TGF-β1 levels was observed (26). However, in the current study, the CSE+Exe group showed a significant increase similar to the control and healthy exercise groups, but it did not surpass the CSE group. TGF-β1 may have different functions at various stages of the disease. It is likely that CSE and TGF-β1 have different signaling mechanisms for regulating ECM proteins. The pathogenesis of COPD involves a complex and intertwined cascade of mediators (17). The study suggests that harmful substances from smoke in the lungs of rats cause irritation and inflammation, outweighing the benefits of anti-inflammatory interventions and antioxidant properties from six weeks of intermittent aerobic exercise. The optimal administration variables, such as intensity, volume, and duration, are crucial in modulating inflammatory mediators and conditions (18). Simultaneously, significant attention is directed towards the nuanced anatomical disparities observed in the pulmonary structures of various animal species compared to those of humans when assessing the findings (24).
In general, cigarette smoke extract (CSE) injections disrupt lung and body structural biotensegrity, leading to pathological conditions. This is due to alterations in the extracellular matrix's elastic properties, affecting elastic fiber performance and causing tissue stiffness. This compromises functional form and energy consumption, reducing flexibility and homeostasis of signaling cascades. The qualitative results of the histological changes in the present study also confirm the destruction of lung epithelial tissue. The CSE control group experienced a significant decrease in elastin due to the activation of the elastase signaling pathways MMP12 and TGF-β1, resulting in a decrease in both the fresh weight and body weight of the rats. Additionally, the speed test in this group yielded significantly lower results than in the control group (27).
Exercise training in the healthy group demonstrated the preservation of elastin elasticity and decreased MMP12 and TGF-β1. This suggests that applying a non-pharmacological training regimen to the rats in the COPD-CS model preserved elastin content compared to the CSE control group by enhancing the signaling pathways of MMP12 and TGF-β1. Additionally, the exercise led to a substantial increase in both lung and body fresh weight, as well as enhanced functional test performance (27). These changes suggest the potential preservation of optimal muscle function and a strengthening of the immune system through anti-inflammatory stimulation, including IL-10, which effectively inhibits the destructive inflammatory pathways in alignment with the dysfunctional signaling pathways in the mentioned extracellular matrix (23).
The type of exercise prescription can significantly influence the achievement of therapeutic goals by optimizing the distribution of stress pressure on the compressive stress components of the cell, thereby controlling the cell's genotype and phenotype and, consequently, its function. It is recommended to analyze the comparison of different or combined sports exercises in isolation and systematically observe the changes in MMP12, a key regulator of elastin tension fibers. This is important due to the diverse behavior of MMPs following different types of mechanical loading on the tissue. However, evaluating the matrix environment, cell behaviors, and force response in a 3D system requires more attention in future research. Additionally, since the variables in the current study were only measured at the serum level, a more comprehensive understanding of the mechanisms governing exercise responses can be achieved by simultaneously examining serum levels and protein expression in lung tissues and other relevant tissues, using samples from both humans and animals.
5.1. Conclusions
The COPD-CS model rats exhibited significant structural and functional lung dysfunction. Intermittent aerobic exercise has been shown to have a favorable effect on lung function. However, further investigation is needed to determine how much it can reverse the modeled damage in the CSE group. Therefore, it is suggested that controlled regular exercise, in the form of a specific prescribed regimen, may have a positive impact on preventing or improving lung health under the mentioned traumatic conditions.