1. Background
Nonalcoholic fatty liver disease (NAFLD) is an emerging global health issue associated with metabolic syndrome, closely linked to obesity, insulin resistance, heightened systemic inflammation, and chronic inflammation. Chronic inflammation is increasingly recognized as a significant factor in the progression of NAFLD and its complications (1). The precise cause of NAFLD has not been fully elucidated (1). The progression mechanism is typically explained by the classic "multiple strikes" theory of NAFLD pathogenesis (1). This theory suggests that lipid accumulation initiates hepatic steatosis, leading to various redox perturbations arising from adipokine secretion, inflammation, lipotoxicity, and disruptions in glucose and lipid metabolism (1, 2). These factors can ultimately progress to non-alcoholic steatohepatitis (NASH) and cirrhosis (2, 3). Cytokines play a crucial role as mediators of inflammation, fibrosis, and cirrhosis in NAFLD (1-4). Prior research has identified many inflammatory mediators, including members of the interleukin-1 (IL-1) cytokine family, which are key contributors to the progression of NAFLD. The pro-inflammatory cytokine interleukin-1β (IL-1β) is particularly significant in this context, playing a pivotal role at all stages of NAFLD, from liver steatosis to NASH and fibrosis. IL-1β is synthesized as an inactive protein and requires proteolytic cleavage to become biologically active (5, 6).
Various pathways may trigger the activation of IL-1β in NAFLD. This cytokine is primarily secreted by activated macrophages and monocytes. It promotes liver steatosis, inflammation, and fibrosis by signaling through the IL-1 receptor, which is widely expressed on several liver cell subpopulations. Interleukin--1β induces hepatic steatosis by increasing triglyceride and cholesterol accumulation in primary liver hepatocytes and promoting the formation of lipid droplets (5-7). Elevated levels of reactive oxygen species (ROS) in mitochondria disrupt the redox balance, triggering an inflammatory response and activating NF-κB (8). Once activated, NF-κB translocates to the nucleus, initiating the transcription of genes such as interleukin-1 beta (8).
Mouse model studies have shown that IL-1β, along with interleukin-6 (IL-6) and TNF, triggers immune responses in the liver, resulting in chronic inflammation. This process impairs the body's defenses by reducing the activity of anti-inflammatory cytokines such as IL-4. Interleukin-4, a multifunctional cytokine primarily produced by activated T cells, mast cells, basophils, and eosinophils, exhibits anti-inflammatory properties by suppressing the production of tumor necrosis factor (TNF)-α and IL-1β (9). Research has shown that IL-4 levels are elevated in non-alcoholic steatohepatitis, potentially increasing the risk of NAFLD (10, 11).
Glucocorticoids have been proposed as a potential treatment for NAFLD across all stages (12). They increase the release of fatty acids from adipose tissue and stimulate their uptake by the liver (12). It is well-documented that plasma triglycerides and free fatty acid (FFA) levels rise in response to glucocorticoids (12). On the other hand, glucocorticoids exert an anti-inflammatory effect by suppressing the expression of inflammatory genes under various inflammatory conditions (12, 13). Glucocorticoids inhibit the production of cytokines, chemokines, adhesion molecules, and other inflammatory proteins, thereby preventing the migration of inflammatory cells to inflamed areas (12, 13).
Additionally, glucocorticoids suppress the production of inflammatory mediators in macrophages and other cells, making them effective in treating various inflammatory diseases caused by immune system dysregulation (14). Studies have shown that neutrophils activated with TNF-α in vitro produce significant amounts of IL-1β, but the production of IL-1β is inhibited by dexamethasone, which dampens the immune response (15).
Metabolic disorders, including NAFLD, are significant factors that disrupt redox homeostasis, inducing metaflammation in liver tissue and systemic blood circulation. Various methods exist to modulate the body's inflammatory state; among the non-invasive approaches, the use of minerals and trace elements (MTEs) is noteworthy. Selenium is an essential mineral that plays a pivotal antioxidant role as part of the structure of antioxidant enzymes, such as selenoproteins (16). Its antioxidant properties help break down peroxides that can disrupt tissue and DNA homeostasis, causing inflammation and associated health issues (17). Selenium supplementation may influence inflammatory processes, glucose regulation, and oxidative stress by suppressing the formation of advanced glycation end products (AGEs) and reducing the production of free radicals and lipid hydroperoxides (18).
In addition, another non-invasive and non-pharmacological approach for improving and regulating inflammation and related disorders is physical exercise. The modulating effects of exercise training on immunological and oxidative stress responses are widely recognized across bodily fluids, organs, and tissues (19). Studies have shown that high-intensity interval training (HIIT) can increase pro-inflammatory cytokines, which subsequently stimulate the production of anti-inflammatory cytokines such as IL-4 (20). High-intensity interval training consists of short, intense efforts followed by periods of rest or low-intensity activity (19, 20) and is recognized as an effective method for enhancing the body's inflammatory and metabolic responses (19, 20).
Hooshangi et al. (2022) demonstrated that a 4-week application of HIIT combined with nanoselenium supplementation resulted in elevated gene expression levels of interleukin-4 and reduced interferon-γ, indicating anti-inflammatory effects (21). Hamedchaman et al. reported that HIIT elicited antioxidant responses in rat testis tissue (19). However, prolonged intensive interval training in various conditions has been shown to negatively impact immune system indices (19).
2. Objectives
3. Methods
In this experimental study, 40 male Wistar rats (aged 6 - 8 weeks, 150 - 190 g) were obtained from the Pasteur Institute of Karaj. The animals were provided food and water ad libitum before and during the experiment. Upon arrival at our laboratory, the rats were given a 1-week acclimation period to adapt to the climate conditions (12: 12 dark/light cycle, 45% - 55% humidity, 24 ± 1°C), with unrestricted access to water and standard chow (composed of 54% mixed carbohydrate, 16% protein, and 30% lipid).
After this acclimation period, the rats were randomly divided into five groups (n = 8 per group): (1) NC, (2) dexamethasone control (DC), (3) dexamethasone + high-intensity interval training + saline (DT), (4) dexamethasone + nano-selenium (DNS), and (5) dexamethasone + high-intensity interval training + nano-selenium (DTS).
Animal care and ethical principles adhered to the Guide for the Care and Use of Laboratory Animals (23), as approved by the Ethics Committee of the Azad University of Sari [IR.IAU.REC.1394.027].
3.1. Training Protocol and Load Measurements
Initially, a one-week acclimation period to treadmill running was conducted prior to the commencement of the training protocol. This acclimation involved 3 sessions per week, with each session lasting 10 minutes. After the familiarization period, the HIIT groups performed running exercises at a velocity of 24 - 34 m/min (estimated as 85 - 100% of VO2max), interspersed with 75-second active rest intervals, on a motorized treadmill (designed by Prof. Ghanbari-Niaki, 14 lanes, Iranian model). The protocol was implemented 6 days a week for 4 weeks (24). Each session included 5 minutes dedicated to warm-up and cool-down phases.
The training protocol followed an incremental intensity trend achieved by gradually increasing treadmill speed and the number of repetitions across the weeks. During the familiarization phase, the animals were conditioned to avoid resting at the end of the treadmill by associating it with an alarm sound, ensuring that the research findings would not be influenced by the possibility of electric shocks.
The exercise program was conducted during the active (dark) cycle. To alleviate cage stress, the sedentary rats were removed from their cages for the same duration as the exercise groups performed their treadmill sessions on a stationary treadmill.
3.2. Nano-Selenium Supplementation
Selenium dioxide was first dissolved into a 2.5 mM solution and combined with 2.5 mM ascorbic acid to synthesize selenium nanoparticles. The resultant mixture was washed using filter paper and centrifuged (25). Mice were administered 100 mg of the stock selenium nanoparticle solution, with a particle size of 250 nm, via gavage every other day. Simultaneously, the healthy control group received a normal saline solution via gavage.
3.3. Nonalcoholic Fatty Liver Disease Induction
Induction of NAFLD was achieved by intraperitoneal injection of 0.4 mg/kg/day dexamethasone (Osweh Corporation, Iran) for three consecutive days (26). The control group received an identical volume of normal saline (NS) solution via intraperitoneal injection over the same three-day period.
3.4. RT-PCR Analysis
In summary, liver tissue was subjected to total RNA isolation using a total RNA extraction mini kit (Denazist, Iran) following the supplier's protocol. The extracted RNA was subsequently treated with the DNase I Kit (Sinaclon, Iran) to eliminate any potential DNA contamination. The RNA samples were then used to synthesize first-strand cDNA using MMLV reverse transcriptase (YTA, Iran), following the manufacturer’s protocol and utilizing an oligo (dT) primer and dNTP mix.
For this investigation, specific primers for IL-4 and IL1β were designed using Primer Premier 5 software. The primer sequences used in this study are provided in Table 1. A real-time polymerase chain reaction (PCR) was performed using RealQ Plus 2x Master Mix Green (Ampliqon, Denmark) on a Rotor-Gene 6000 instrument (Corbett Research, Australia). The thermal cycling conditions included an initial temperature of 95°C for 15 minutes, followed by 40 cycles, each consisting of 30 seconds at 57°C and 30 seconds at 72°C.
| Gene and Reverse and Forward Primer | Amplicon Size | NCBI Accession Number |
|---|---|---|
| IL-1β | 100 | NM_001130538.1 |
| F:5’-CTCCATGAGCTTTGTACAAGG-3’ | ||
| R:5’-TGCTGATGTACCAGTTGGGG-3’ | ||
| IL-4 | 165 | NM_001011952.1 |
| F-5'-CAAGGAACACCACGGAGAAC-3' | ||
| R-5'-TCTTCAAGCACGGAGGTACA-3' | ||
| GAPDH | 147 | NM_012520.2 |
| F-5'-CAAGTTCAAGGGCACAGTCA-3' | ||
| R-5'-CCCCATTTGATGTTAGCGGG-3' |
Abbreviation: IL, interleukin.
The expression level of each sample was standardized using GAPDH as an internal control. The relative expression levels were determined using the comparative CT technique (2−ΔΔCT) as described by Schmittgen (27).
3.5. Statistical Analysis
The data were presented as means ± SD. One-way ANOVA and Bonferroni post-hoc tests were respectively used for inter-group and intra-group comparisons. Statistical significance was set at P < 0.05. The statistical analysis was performed using the SPSS statistical program (Version 22).
4. Results
4.1. Final Body Weight and Weight Changes
The results of 4 weeks of aerobic training combined with nano-selenium supplementation showed a significant difference in the final weights of the study subjects (P = 0.033). Additionally, dexamethasone injection led to a decrease in body weight in the DC group compared to the NC group (P = 0.003). All interventions were able to modulate the final body weight in relation to the NC group (DT: P = 0.0046; DS: P = 0.0014; and DST: P = 0.0007) (Table 2).
Abbreviations: NC, normal control; DC, dexamethasone control; DT, dexamethasone + high-intensity interval training + saline; DS, dexamethasone + selenium supplement; DST, dexamethasone + selenium supplement + high-intensity interval training.
a Values are expressed as mean ± SD.
b Versus the NC group.
c Versus the DC group (P < 0.05).
d Significant group difference at P < 0.05.
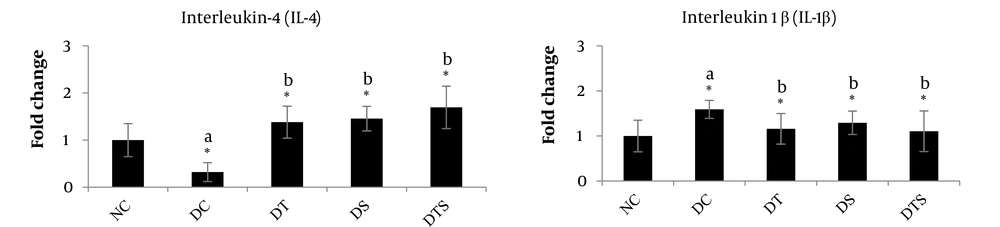
The results of ANOVA indicated that 4 weeks of high-intensity interval training and nano-selenium supplementation combined with dexamethasone injection resulted in a significant difference in IL-4 (P = 0.077) and IL-1β (P = 0.029) gene expression levels among the study groups (Table 3).
| Variables | Normal Control | Dexamethasone Control | Dexamethasone + High Intensity Interval Training | Dexamethasone + Nano-Selenium | Dexamethasone + High Intensity Interval Training + Nano-Selenium | P-Value b |
|---|---|---|---|---|---|---|
| IL-1β | 1.008 ± 0.291 | 1.280 ± 0.458 | 1.310 ± 0.505 | 1.018 ± 0.865 | 1.440 ± 0.450 | 0.029 |
| IL-4 | 1.007 ± 0.269 | 1.143 ± 0.401 | 1.300 ± 1.094 | 1.576 ± 0.864 | 1.376 ± 0.162 | 0.077 |
Abbreviation: IL, interleukin.
a Values are expressed as mean ± SD.
b Significant group difference at P < 0.05 according to ANOVA.
The results of the Bonferroni post-hoc test revealed that dexamethasone injection led to an increase in interleukin-1β (P = 0.0012) and a reduction in interleukin-4 (P = 0.0173) in the liver tissue of the DC group compared to the NC group. All interventions effectively up-regulated interleukin-4 (DT: P = 0.0014; DS: P = 0.0046; DTS: P = 0.0007) and modulated interleukin-1β (DT: P = 0.0014; DS: P = 0.0046; DTS: P = 0.0007) in comparison to the DC group. Additionally, HIIT combined with nano-selenium supplementation showed the most significant effects on modulating interleukin-4 and interleukin-1β levels, bringing them closer to the NC levels (Figure 1).
5. Discussion
5.1. Effects of Aerobic Exercise and/with Selenium Supplementation on Body Weight
Impaired glucose homeostasis is well known to lead to insulin resistance, which affects body weight through altered fat distribution and subsequent fat accumulation (19). Dexamethasone injection, in its initial stages, has been shown to cause hyperinsulinemia and an enlargement of the coronary lipoprotein lipase (LPL) pool, leading to excessive triglyceride storage (28). However, this storage is taken up by the heart and skeletal muscle, as no evidence of free fatty acids has been observed in the blood circulation (28). On the other hand, chronic administration of dexamethasone has been associated with mesenteric white adipose tissue accumulation (29). Additionally, both acute and chronic systemic injections of dexamethasone can cause hyperglycemia (severe enough to induce ketoacidosis) in both diabetic and non-diabetic patients (30, 31).
Despite findings from other studies (32, 33), animals injected with 0.4 mg/kg of dexamethasone for 3 days in this study did not exhibit significant weight gain but instead showed a notable drop in weight. This finding aligns with the studies of Pasternak et al., Lukins and Manninen, and Alan I and Alan B (34-36). The probable mechanism behind this observation could be attributed to the metaboregulatory effects of leptin. Leptin, a stress-related hormone, is stimulated by stress and is known to restrict food intake, suppress feeding, and subsequently increase energy expenditure, resulting in body weight loss in animal models (37, 38). However, the lack of leptin assessment in the present study is a limitation.
Another possible mechanism for the observed weight loss may involve leptin-induced 5-HT (serotonin) upregulation. This mimics the effects of selective serotonin reuptake inhibitors (SSRIs), inducing satiety and reducing appetite (39). This mechanism may explain how dexamethasone led to weight loss in the animal models of the present study. Unfortunately, 5-HT levels were not measured in this study, and no conclusions can be drawn on this basis.
In the current study, we observed that aerobic exercise and nano-selenium supplementation prevented body weight loss and modulated the probable HOMA-IR induction despite the presence of dexamethasone injection. Endothelial dysfunction is known to be associated with insulin resistance, primarily through a reduction in nitric oxide secretion from endothelial cells. Insulin, via nitric oxide-dependent mechanisms, facilitates glucose uptake in skeletal muscle (19). Aerobic exercise appears to induce anabolic adaptations through the activation of the endothelial nitric oxide synthase (eNOS) enzyme, leading to improved endothelial function, possibly through contraction-induced shear stress. Additionally, aerobic exercise exerts anti-catabolic effects that prevent muscle atrophy and promote muscle hypertrophy (19), potentially counteracting dexamethasone-induced catabolic pathways.
Aerobic exercise also contributes to metabolic adaptations, particularly its glucoregulatory effects, by upregulating GLUT-4 expression in blood circulation and ameliorating systemic hyperglycemia (19). However, blood glucose and insulin levels were not assessed in the present study, which is another limitation.
The modulatory effect of nano-selenium supplementation on muscle atrophy may be attributed to the anti-aging properties of these trace elements (40). It has been shown that selenoprotein supplementation promotes muscle fiber hypertrophy by reducing endoplasmic reticulum stress (41, 42). Additionally, selenium plays a critical role in the structure of antioxidants and is essential for reactive oxygen and nitrogen species (RONS) scavenging (40, 43). The prevention of muscle atrophy induced by dexamethasone injection observed in this study may be due to the potentially anti-aging and ergogenic effects of selenium supplementation.
5.2. Effects of Aerobic Exercise and/with Selenium Supplementation on Gene Expression Levels of IL4 and IL1β
The results showed that the expression of the IL-4 gene in the intense interval exercise group and the selenium supplement group differed compared to the interaction group of strenuous exercise and selenium supplementation. The reduction in IL-4 gene levels in the liver tissue of rats indicates the immunosuppressive effects of dexamethasone injection. It can be concluded that selenium supplementation and the combination of exercise and selenium supplementation both play a significant role in enhancing immune system efficiency. Studies have shown that selenium exhibits various effects, including antioxidant and anti-inflammatory properties (44). Selenium may promote lymphocyte proliferation by reducing oxidative stress and enhancing free thiols (16-18, 44). Reports indicate that selenium induces the proliferation of precursor cytotoxic cells, increases the number of B and T cells, eliminates oxidative factors, and may consequently reduce levels of inflammatory cytokines (45). However, the lack of measurement of oxidative stress-related factors in this study is a limitation of the current research.
Exercise intensity predominantly exerts its effects on immune system markers (46). Evidence has shown that physical activity improves immune system function (46, 47). However, the precise mechanisms underlying these effects are not yet fully understood. Improved immune function as a result of exercise may represent one of the most important benefits of physical activity.
The results of the current study suggest that intense interval exercise and selenium supplementation may individually regulate the gene expression levels of interleukin-4 (IL-4) and interleukin-1β (IL-1β) in the liver tissue of dexamethasone-induced rats. It was demonstrated that intense interval exercise led to a significant reduction in IL-4 gene expression, which could be explained by the enhanced synthesis and secretion of pro-inflammatory cytokines that promote lymphocyte differentiation, thereby suppressing immunological responses and anti-inflammatory cytokines.
The study also revealed that four weeks of selenium supplementation and intense interval exercise resulted in significant changes in the gene expression levels of IL-1β, while the combination of selenium supplementation and intense interval exercise had the greatest effects on IL-1β gene expression. Interleukin-1β is a key cytokine that promotes inflammation and initiates several physiological reactions, including fever, lymphocyte activation, and the production of acute-phase proteins (5, 6). It enhances T cell function, which is crucial for the adaptive immune response, and promotes fibroblast growth and collagen synthesis, leading to chronic inflammatory changes (7).
The inflammatory response is closely associated with increased ROS in mitochondria (8). Elevated ROS levels within the mitochondria alter the redox state of NF-κB, triggering its activation and subsequent inflammatory responses by stimulating the kinase pathway (7, 8). Once activated, NF-κB translocates to the nucleus and stimulates the expression of specific genes, including IL-1β and IL-6. Macrophages and monocytes primarily produce IL-1β and IL-6, which play vital roles in immunological, inflammatory, and hematological responses. IL-1β directly stimulates mature immune cells to produce pro-inflammatory cytokines, such as IL-6 (5-8). The features of physical activity, such as intensity and volume, are key determinants of the extent of short-term responses and chronic adaptations to exercise. For instance, one study found that 16 weeks of exercise significantly reduced IL-1β levels (32); however, other research has shown that exercise may have no effect on inflammatory mediators like IL-1β (22). Moreover, long-term intense interval exercise has been reported to negatively impact immune system indicators (22). In the present study, four weeks of intense interval exercise led to a reduction in IL-1β gene expression levels in the liver tissue of dexamethasone-induced animal models. This suggests a possible mechanism involving the activation of the antioxidant system through strenuous interval exercise, thereby reducing pro-inflammatory cytokine levels.
One limitation of the current study is the lack of assessment of related proteins using the western blot technique, which could have enhanced the generalizability of the study's results.
5.3. Conclusions
The findings of the current study provide conflicting evidence: While consuming nano-selenium supplementation may enhance anti-inflammatory benefits, adapting to high-intensity interval training may prevent the disruption of redox equilibrium in gene expression in liver tissues. Given that inflammation and cytokine imbalance contribute to various health problems, these findings could aid in developing new approaches to addressing health and wellness challenges. Notably, selenium-rich supplementation combined with a high-intensity interval training protocol showed the most significant effect in enhancing IL-4 expression and down-regulating IL-1β gene expression, overall suggesting its potential as an effective method for modulating inflammatory conditions.