1. Background
Sepsis is one of the most critical healthcare-associated infections, with a significant mortality rate across all communities (1). Any delay in initiating aggressive antibiotic therapy is unacceptable (2). Unfortunately, multidrug-resistant bacterial agents, as causes of this disease, are increasing globally, and Iran is no exception (3, 4).
Because bacterial sensitivity results are reported with several days’ delay and are often negative due to prior antibiotic use or limitations in microbial culture technology, antibiotic treatment is typically started empirically, based on antibiotic sensitivity patterns from similar regional studies (1-5). Numerous studies have been conducted worldwide on the antibiotic sensitivity of agents causing nosocomial sepsis (6-15). The strength of these studies is the precise definition of hospital-acquired infection (6-12, 16-18). However, these studies have two primary limitations: First, the inclusion of patients without evidence of sepsis (6-18) and second, the failure to exclude cases with positive cultures from contaminating bacteria in the final analysis (7, 9, 11-13). Due to these limitations, the findings of previous studies may have limited applicability in the treatment of patients with nosocomial sepsis.
2. Objectives
The present study examines bacterial pathogens causing hospital sepsis and their antibiotic sensitivity in Isfahan, Iran. These results may enable more effective selection of antibiotics for the initial treatment of nosocomial sepsis patients in this region.
3. Methods
3.1. Study Design
This cross-sectional study was conducted across three referral hospitals in Isfahan, Iran: Al-Zahra, Dr. Shariati, and Dr. Gharazi Medical Centers. Clinical information and microbiological results were collected by trained infection control nurses and laboratory personnel at these hospitals. Each hospital's microbiology laboratory holds a quality certificate from the Iranian Ministry of Health and collaborates with the World Health Organization as part of the Global Antimicrobial Resistance Surveillance System (GLASS) program (16).
3.2. Specimen Collection
All hospitalized patients with positive blood culture results were included in the study. Patients who did not meet the sepsis criteria or who had community-acquired infections were excluded. Additionally, patients with blood cultures that grew contaminant bacteria were excluded.
Sepsis was defined as meeting at least two of the following criteria, with either the first or second criterion being mandatory: Temperature > 38.0°C or < 36.0°C, unexplained tachycardia, unexplained tachypnea, WBC count ≥ 12,000/µL in adults and ≥ 15,000/µL in children, or ≤ 4,000/µL, and hypotension (16).
Nosocomial infection was defined as the growth of bacteria in a culture medium from a patient after the 48th hour of hospitalization, coinciding with new signs of infection, such as fever, tachypnea, tachycardia, or hypotension (16).
The growth of a microorganism that is uncommon as a causative agent of sepsis was considered a contaminant when it grew only once from two or more culture samples taken from the patient (16).
Common sepsis-causing organisms included enteric gram-negative bacteria (Escherichia coli, Klebsiella spp., Proteus spp., Enterobacter spp., and Salmonella spp.), Pseudomonas aeruginosa, Acinetobacter spp., Enterococcus spp., Staphylococcus aureus, Group A Streptococcus, Streptococcus pneumonia, Haemophilus influenzae type b, and Neisseria meningitidis. Blood culture samples were collected using standard aseptic techniques and promptly transported to the laboratory for analysis.
3.3. Bacterial Isolation
Bacterial isolation was performed by incubating blood samples in either Bactec Alert System medium or conventional blood culture medium for 72 hours. Identification of microorganisms and determination of their antibiotic sensitivity followed the guidelines of the Clinical Laboratory and Standards Institute (17). Sensitivity of isolates was assessed for the following classes of antibiotics: Aminoglycosides (gentamicin 10 µg or amikacin 10 µg), cephalosporins (cefotaxime 30 µg or ceftriaxone 30 µg, ceftazidime 30 µg, and cefepime 30 µg), fluoroquinolones (ciprofloxacin 5 µg or levofloxacin 5 µg), folate inhibitors (trimethoprim-sulfamethoxazole 1.25/23.75 µg), carbapenems (meropenem 10 µg), glycopeptides (vancomycin 30 µg), penicillins (ampicillin 10 µg), and oxazolidinones (linezolid 30 µg). Dehydrated antibiotic discs were commercially sourced from MAST, Merseyside, UK. Minimum inhibitory concentrations (MICs) for vancomycin and colistin were determined using E-test strips (Liofilchem, Roseto Degli Abruzzi, Italy) per the manufacturer's instructions. All test methods and kits were standardized across partner laboratories (16).
3.4. Statistical Analysis
The list of bacterial agents causing sepsis, along with their antibiotic sensitivity profiles, patient age, and gender, was extracted from WHONET v5.6 software across the enrolled hospitals. Data analysis was conducted using SPSS version 18.0, employing chi-square and Fisher's exact tests for statistical evaluation. A P-value of less than 0.05 was considered statistically significant.
3.5. Ethical Consideration
The study protocols received approval from the Research Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.944). The findings were derived from patient data collected for treatment purposes upon hospital admission, with strict adherence to patient anonymity throughout the study. Consequently, obtaining informed consent from the patients was not required.
4. Results
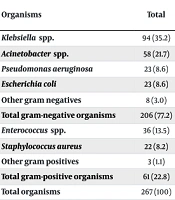
A total of 267 patients diagnosed with nosocomial sepsis were identified, of whom 146 (54.7%) were male, and 39 (14.6%) were under 20 years of age. Gram-negative bacteria accounted for 206 (77.2%) of the isolates, with Klebsiella spp. (35.2%) being the predominant pathogen, followed by Acinetobacter spp. (21.7%), E. coli (8.6%), P. aeruginosa (8.6%), and other gram-negative rods (3.0%). The frequency of gram-negative bacteria was consistent across different age and gender groups (Table 1).
| Organisms | Total | Gender | Age Group (y) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P-Value | OR (95% CI) | < 20 | > 20 | P-Value | OR (95% CI) | ||
| Klebsiella spp. | 94 (35.2) | 56 (38.4) | 38 (31.4) | 0.236 | 0.736 (0.442 - 1.224) | 16 (41.0) | 78 (34.2) | 0.410 | 0.748 (0.373 - 1.497) |
| Acinetobacter spp. | 58 (21.7) | 28 (19.2) | 30 (24.8) | 0.268 | 1.389 (0.775 - 2.489) | 11 (28.2) | 47 (20.6) | 0.288 | 0.661 (0.307 - 1.424) |
| Pseudomonas aeruginosa | 23 (8.6) | 14 (9.6) | 9 (7.4) | 0.533 | 0.758 (0.316-1.816) | 4 (10.3) | 19 (8.3) | 0.692 | 0.795 (0.255-2.477) |
| Escherichia coli | 23 (8.6) | 11 (7.5) | 12 (9.9) | 0.490 | 1.351 (0.574 - 3.181) | 2 (5.1) | 21 (9.2) | 0.546 | 1.877 (0.422 - 8.344) |
| Other gram negatives | 8 (3.0) | 3 (2.1) | 5 (4.1) | 0.474 | 2.055 (0.481 - 8.778) | 0 (0.0) | 8 (3.5) | 0.608 | - |
| Total gram-negative organisms | 206 (77.2) | 112 (76.7) | 94 (77.7) | 0.850 | 1.057 (0.595 - 1.878) | 33 (84.6) | 173 (75.9) | 0.230 | 0.572 (0.228 - 1.437) |
| Enterococcus spp. | 36 (13.5) | 20 (13.7) | 16 (13.2) | 0.910 | 0.960 (0.474 - 1.946) | 5 (12.8) | 31 (13.4) | 0.896 | 1.070 (0.389 - 2.945) |
| Staphylococcus aureus | 22 (8.2) | 14 (9.6) | 8 (6.6) | 0.378 | 0.668 (0.270 - 1.649) | 1 (2.6) | 21 (9.2) | 0.218 | 3.885 (0.503 - 29.520) |
| Other gram positives | 3 (1.1) | 0 (0.0) | 3 (2.5) | 0.092 | - | 0 (0.0) | 3 (1.3) | > 0.99 | - |
| Total gram-positive organisms | 61 (22.8) | 34 (23.3) | 27 (22.3) | 0.850 | 0.946 (0.533 - 1.681) | 6 (15.4) | 55 (24.1) | 0.230 | 1.749 (0.696 - 4.393) |
| Total organisms | 267 (100) | 146 (54.7) | 121 (45.3) | - | - | 39 (14.6) | 228 (85.4) | - | - |
Frequency of Bacteria Causing HCA-Sepsis in Patients Hospitalized in Three Hospitals According to Sex and Age Groups in Isfahan, Iran a
The sensitivity of these bacteria to the antibiotics tested was as follows: Colistin (100%), amikacin (49.7%), meropenem (40.0%), ciprofloxacin (31.8%), trimethoprim-sulfamethoxazole (31.4%), cefotaxime/ceftriaxone (30.0%), ceftazidime (23.5%), and cefepime (19.7%). The sensitivity rates to different antibiotics were generally similar across age and sex categories, except for amikacin, which was more effective in patients under 20 years of age (53.3% vs. 30.0% in the under-20 age group, P = 0.02) (Table 2).
| Antibiotic | Total | Gender | Age Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n/N a) | Female (n/N) | P-Value | OR (95% CI) | < 20 (n/N a) | > 20 (n/N a) | P-Value | OR (95% CI) | ||
| Ceftazidime | 47/200 (23.5) | 23/108 (21.3) | 24/92 (26.1) | 0.426 | 1.304 (0.678 - 2.511) | 4/33 (12.1) | 43/167 (25.7) | 0.116 | 2.514 (0.836-7.564) |
| Cefotaxime or ceftriaxone | 12/40 (30.0) | 4/20 (20.0) | 8/20 (40.0) | 0.301 | 2.667 (0.648 - 10.972) | 0/4 (0.0) | 12/36 (33.3) | 0.297 | - |
| Cefepime | 39/198 (19.7) | 22/107 (20.6) | 17/91 (18.7) | 0.740 | 0.888 (0.438 - 1.797) | 4/32 (12.5) | 35/166 (21.1) | 0.337 | 1.870 (0.615 - 5.687) |
| Meropenem | 78/195 (40.0) | 43/103 (41.7) | 35/92 (38.0) | 0.598 | 0.857 (0.482 - 1.522) | 15/28 (53.6) | 63/167 (37.7) | 0.113 | 0.525 (0.235 - 1.175) |
| Amikacin | 99/199 (49.7) | 60/106 (55.7) | 40/93 (43.0) | 0.056 | 0.579 (0.330 - 1.015) | 9/30 (30.0) | 90/169 (53.3) | 0.019 | 2.658 (1.151 - 6.141) |
| Ciprofloxacin | 48/151 (31.8) | 28/86 (32.6) | 20/65 (30.8) | 0.815 | 0.921 (0.460 - 1.842) | 3/7 (42.9) | 45/144 (31.2) | 0.680 | 0.606 (0.130 - 2.821) |
| levofloxacin | 13/53 (24.5) | 7/24 (29.2) | 6/29 (20.7) | 0.475 | 0.634 (0.180 - 2.229) | 0/4 (0.0) | 13/49 (26.5) | 0.561 | - |
| Trimethoprime-sulfamethoxazole | 32/102 (31.4) | 15/55 (27.3) | 17/47 (36.2) | 0.334 | 1.511 (0.652 - 3.501) | 4/15 (26.7) | 28/87 (32.2) | 0.771 | 1.305 (0.382 - 4.463) |
| Colistin | 67/67 (100) | 33/33 (100) | 34/34 (100) | - | - | 10/10 (100) | 57/57 (100) | - | - |
Antimicrobial Sensitivity of Gram-Negative Isolates Causing HCA-sepsis in Patients Hospitalized in Three Hospitals According to sex and age Groups in Isfahan, Iran
In the present study, Enterococcus spp. was the most common gram-positive pathogen causing sepsis (13.5%), followed by S. aureus (8.2%) and other gram-positive bacteria (1.1%). The frequency of antibiotic sensitivity among gram-positive bacteria remained consistent across different age and sex groups (Table 1).
Linezolid was the most effective antibiotic for treating gram-positive bacteria responsible for nosocomial sepsis (100%), followed by gentamicin (70.0%), vancomycin (35.1%), clindamycin (25.6%), ampicillin (24.3%), and ciprofloxacin (22.7%). Sensitivity rates of gram-positive isolates to the tested antibiotics were similar across various age and sex categories (Table 3).
| Antibiotic | Total | Gender | Age Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n/N a) | Female (n/N) | P-Value | OR (95% CI) | < 20 (n/N a) | >20 (n/N a) | P-Value | OR (95% CI) | ||
| Vancomycin | 26/74 (35.1) | 15/40 (37.5) | 11/34 (32.3) | 0.644 | 0.797 (0.305 - 2.087) | 1/9 (11.1) | 25/65 (38.5) | 0.147 | 5.000 (0.589 - 42.415) |
| Clindamycin | 10/39 (25.6) | 6/21 (28.6) | 4/18 (22.2) | 0.726 | 0.714 (0.166 - 3.075) | 1/4 (25.0) | 9/35 (25.7) | 1.000 | 1.038 (0.095 - 11.296) |
| Gentamicin | 14/20 (70) | 8/13 (61.5) | 6/7 (85.7) | 0.354 | 3.750 (0.342 - 41.081) | 0/0 (0.0) | 14/20 (70.0) | - | - |
| Ciprofloxacin | 10/44 (22.7) | 6/26 (23.1) | 4/18 (22.2) | 1.000 | 0.952 (0.226 - 4.011) | 1/2 (50.0) | 9/42 (21.4) | 0.407 | 0.273 (0.015 - 4.801) |
| Ampicillin | 9/37 (24.3) | 5/20 (25.0) | 4/17 (23.5) | 1.000 | 0.923 (0.204 - 4.179) | 1/5 (20.0) | 8/32 (25.0) | 1.000 | 1.333 (0.129 - 13.743) |
| Linezolid | 31/31 (100) | 16/16 (100) | 15/15 (100) | - | - | 5/5 (100) | 26/26 (100) | - | - |
Antimicrobial Sensitivity of Gram-Positive Isolates Causing HCA-Sepsis in Patients Hospitalized in Three Hospitals According to sex and age Groups in Isfahan, Iran
5. Discussion
This investigation revealed that the most common causes of nosocomial sepsis were Klebsiella spp., Acinetobacter spp., Enterococcus spp., E. coli, P. aeruginosa, and S. aureus. Additionally, colistin and linezolid were found to be the most effective antibiotics against gram-negative and gram-positive organisms causing this disease, respectively. High resistance of gram-negative bacteria to other tested antibiotics, including third- and fourth-generation cephalosporins, meropenem, amikacin, ciprofloxacin, and trimethoprim-sulfamethoxazole, and significant insensitivity of gram-positive organisms to vancomycin, clindamycin, ampicillin, and ciprofloxacin, were observed. Furthermore, moderate sensitivity of gram-positive bacteria to gentamicin was noted.
This investigation represents the first study examining the antibiotic sensitivity of microbial agents causing nosocomial sepsis in this under-researched area. Previous studies in Iran primarily focused on bacterial agents responsible for bloodstream infections without excluding community-acquired cases (13). Unlike prior studies, this study excluded contaminant bacterial agents that grew in the patients' blood cultures by applying defined criteria (12, 13).
The most frequent causes of nosocomial sepsis in our study were Klebsiella spp., Acinetobacter spp., Enterococcus spp., E. coli, P.aeruginosa, and S. aureus, respectively. These microorganisms have been consistently reported as primary causes of nosocomial bloodstream infections in hospitalized patients (6-11, 14, 15). Unlike our findings, the absence of strict diagnostic criteria to exclude confounding skin-colonizing agents has led some prior investigations to classify coagulase-negative Staphylococci as a primary cause of nosocomial bloodstream infection (7, 9, 11, 12). In this study and several others, two separate positive blood cultures were required to identify skin flora, such as coagulase-negative Staphylococci, as the causative agent of bloodstream infection (6, 8, 10, 14, 15).
The order of the most common bacterial causes of nosocomial sepsis varies across geographic regions. While Klebsiella spp. was the leading cause of hospital-acquired infections in our study as well as in studies from Egypt, Europe, and Taiwan (9, 14, 15), S. aureus was predominant in Brazil and Japan (6, 8), E. coli in China (10), and Enterococcus spp. in Estonia (7).
The present investigation demonstrated that gram-negative bacilli causing nosocomial sepsis exhibit very high resistance to most antibiotic classes studied, indicating that these antibiotics cannot be relied upon for treating this life-threatening infection. Fewer than 40% of gram-negative bacteria in this study were sensitive to ceftazidime, cefotaxime/ceftriaxone, cefepime, meropenem, ciprofloxacin, levofloxacin, and trimethoprim-sulfamethoxazole, and fewer than 50% to amikacin. Consequently, these antibiotics are not suitable empirical choices for treating patients with nosocomial sepsis.
The low antibiotic sensitivity of gram-negative bacteria in our study to third- and fourth-generation cephalosporins, carbapenems, fluoroquinolones, aminoglycosides, and trimethoprim-sulfamethoxazole aligns with findings from a previous study in Iran (13). However, studies conducted in China, Egypt, Brazil, and Taiwan reported higher antibiotic susceptibility of these bacteria to carbapenems, fluoroquinolones, and aminoglycosides (8-11, 14, 15). Conversely, in a study from Estonia, low antibiotic resistance of these organisms to most of the examined antibiotics, including third-generation cephalosporins, carbapenems, fluoroquinolones, and aminoglycosides, was observed (7).
Variations in antibiotic resistance patterns across regions may depend on each society's economic, social, and environmental factors. Implementing Antibiotic Stewardship Programs and other infection control measures in hospitals are among the most effective strategies for preventing antibiotic resistance development in any geographical area.
In the present study, the sensitivity of gram-negative bacteria causing sepsis among individuals over 20 years of age (53.3%) differed significantly from those under 20 years (30.05%). However, given the low sensitivity of these microorganisms to the drug across both age groups, this finding holds limited clinical significance.
Our study showed that all gram-negative organisms responsible for this infection were sensitive to colistin. This antibiotic belongs to the polymyxin class, originally introduced years ago for microbial infections (18). However, its use was quickly abandoned due to high renal toxicity and limited therapeutic efficacy (19). With numerous global reports now highlighting resistance of gram-negative bacteria to all available antibiotics—especially in nosocomial infections colistin has re-emerged as a last-line treatment option in combination therapy (18, 20).
This investigation, consistent with previous studies, demonstrated high sensitivity of gram-positive bacteria to linezolid (6, 10, 11, 14, 15), moderate sensitivity to gentamicin (6, 10), and low sensitivity to clindamycin, ampicillin, and ciprofloxacin (7, 9, 10, 12, 13). However, in contrast to most prior studies (6, 7, 9-11, 14, 15), the sensitivity of these organisms to vancomycin in this study was low. The high resistance of gram-positive bacteria to most of the tested antibiotics, particularly vancomycin, and the reliable sensitivity to only one last-line therapeutic option is a concerning observation. This underscores the potential for a serious challenge in treating these infections if new antibiotics are not developed in the coming years.
This study faced several limitations. First, only three major hospitals in the area were included, which limits the generalizability of the findings to other medical centers. Future investigations should consider evaluating additional hospitals for broader applicability. Second, the underlying conditions of patients with nosocomial infections were not examined. Understanding these conditions could assist physicians in selecting more tailored initial treatments based on varied patient profiles. Lastly, the antibiotic sensitivity tests in this study were conducted using the antibiotic discs and strips available during routine laboratory procedures. Testing all bacterial isolates against the full range of studied antibiotics could yield more precise results.
Our findings indicate that Klebsiella spp., Acinetobacter spp., Enterococcus spp., E. coli, P. aeruginosa, and S. aureus were the most common pathogens associated with nosocomial sepsis in Isfahan province. The antibiotic sensitivity tests revealed high resistance among these bacterial agents to most of the antibiotics tested. Most gram-negative bacteria were only sensitive to colistin, and gram-positive bacteria showed high sensitivity exclusively to linezolid.