1. Background
In recent years, advances in the treatment of malignancies have significantly improved patient survival rates. However, these therapeutic advancements have brought new challenges, particularly regarding the side effects that impact the quality of life of cancer patients (1). Among these complications, oral mucositis is one of the most severe side effects encountered by patients undergoing radiotherapy or chemotherapy.
Studies indicate that approximately 5 - 15% of patients receiving standard chemotherapy protocols, and all patients undergoing radiotherapy with cumulative doses exceeding 50 Gy, develop oral mucositis (2). The occurrence of oral mucositis is influenced by various factors, including the type of treatment and the sensitivity of individual patients (3). Patients receiving combined chemotherapy and radiotherapy for head and neck tumors, or those undergoing high-dose chemotherapy before bone marrow transplantation, are at a higher risk of developing severe oral mucositis.
The primary biological cause of mucositis is the direct toxicity of chemotherapy or radiotherapy, although secondary factors such as salivary gland dysfunction, local trauma, infections (both local and systemic), and other disruptions also play a role (4, 5).
Oral mucositis leads to severe, debilitating pain that significantly increases the morbidity associated with cancer treatments. The pain caused by mucositis often necessitates opioid pain management and, due to difficulties with oral intake, can require intravenous or enteral feeding. Severe mucositis can also disrupt treatment plans, sometimes leading to the suspension of therapy and a subsequent reduction in patient survival.
In addition to the physical discomfort, mucositis is associated with secondary complications such as nausea, vomiting, diarrhea, and significant quality-of-life decline, including sleep disturbances, anorexia, and weight loss. It also increases the length of hospital stays and the need for specialized interventions (4).
Oral mucositis is observed in 40% of adult patients receiving standard chemotherapy doses but is even more common in children, with a prevalence of 65% among pediatric cancer patients undergoing chemotherapy (6, 7).
2. Objectives
Given the prevalence of oral mucositis as a common complication of chemotherapy, this study investigates its prevalence and associated factors in children with malignancies undergoing chemotherapy.
3. Methods
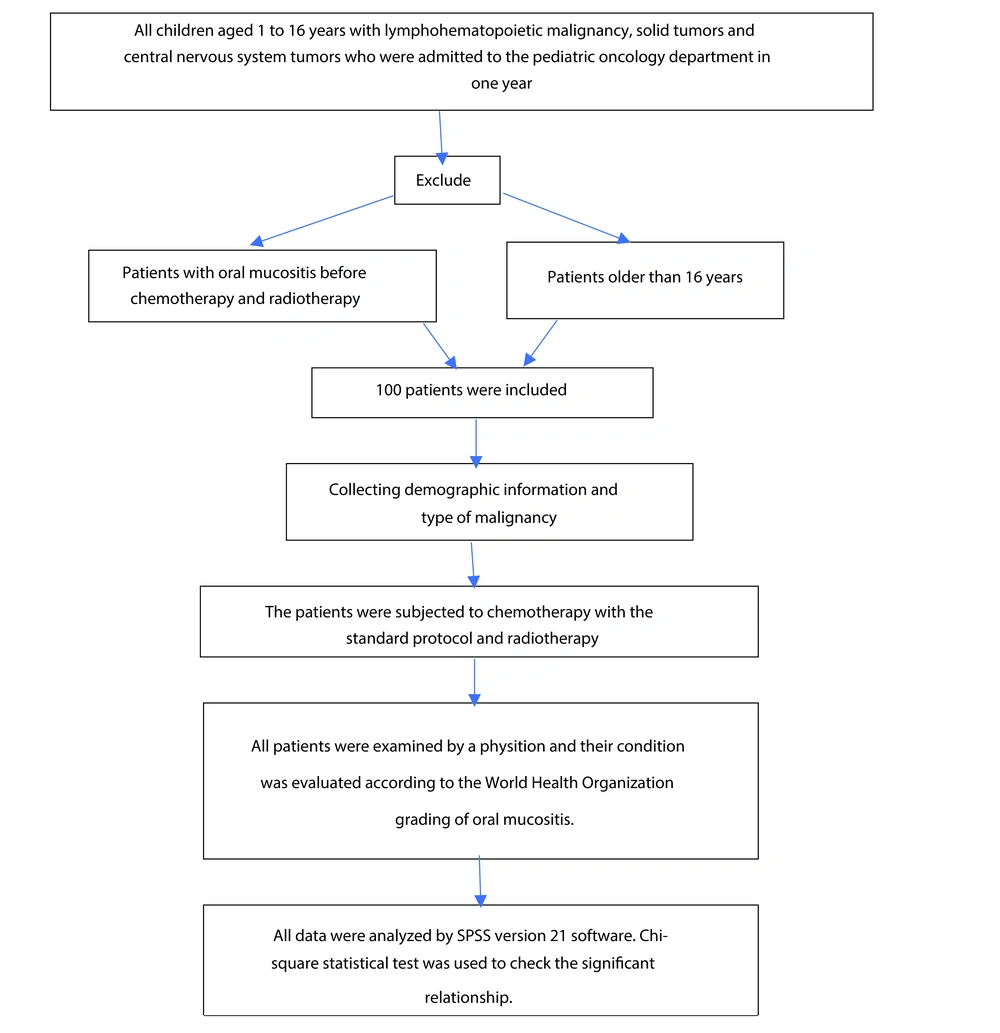
This descriptive study was prospective, and all children aged 1 to 16 years with lymphohematopoietic malignancy, solid tumors, and central nervous system tumors who were admitted to the Pediatric Oncology Department of Aliebnabitaleb Hospital of Zahedan University of Medical Science in one year due to mucositis were included in the study. This hospital is the central and referral hospital for pediatric cancer patients in Southeast Iran. Patients with acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML), Hodgkin's and non-Hodgkin's lymphoma, rhabdomyosarcoma, Ewing's sarcoma, osteosarcoma, Wilms' tumor, neuroblastoma, and brain tumor in the age range of 1 to 16 years were included in the study. The University Ethics Committee, with the code IR.ZAUMS.REC.1397.044, approved this study.
Patients with oral mucositis before chemotherapy and radiotherapy and older than 16 years were excluded from the study, and 100 patients were included in the study. Demographic information, including age, gender, height, weight, and Body Mass Index, was collected. Also, the type of malignancy and grade of mucositis were recorded based on the World Health Organization (WHO) Scale. Mucositis can be measured by clinical toxicity scales such as Mucositis General Assessment Scales, several Mucositis Variable Scales, and Treatment-Specific Scales. From the most frequently used scale is the WHO, which classifies the severity of lesions into 4 degrees (8).
Before starting chemotherapy and radiotherapy, all patients were examined by a physician, and their condition was evaluated according to the WHO grading of oral mucositis.
Therefore, a score of 0 was considered for people without mucositis, and a score of 1 was considered for erythema without ulceration. Also, for the cases of erythema with ulcers, where the patient was able to eat solids, a score of 2 was given; a score of 3 was given for ulcer patients, where the patient was only able to eat liquids; and a score of 4 was considered for patients who were unable to eat food (Table 1) (8).
| Variable | Description |
|---|---|
| Grade | The World Health Organization grades of oral mucositis |
| 0 | No changes |
| I | Soreness/erythema |
| II | Soreness/erythema + ulceration + ability to eat solid foods |
| III | Soreness/erythema + ulceration + ability to use a liquid diet only |
| IV | Soreness/erythema + ulceration + no possible oral alimentation |
The patients were subjected to chemotherapy with the standard protocol, and after completion of chemotherapy and radiotherapy, they were examined for mucositis.
After completing the data collection, descriptive statistics, including percentage, frequency, mean, and standard deviation, were used to describe the data. All data were analyzed by SPSS version 21 software. Chi-square statistical test was used to check the significant relationship. (Figure 1).
4. Results
Out of 100 patients with mucositis, 61 (61%) were boys and 39 (39%) were girls. The mean age of the studied patients was 7.84 ± 3.93 years. The largest number of patients with mucositis were ALL patients, comprising 64 patients (64%). Twelve patients (12%) with mucositis were AML, and 24 patients (24%) had other malignancies, such as Hodgkin's lymphoma, non-Hodgkin's lymphoma, neuroblastoma, and central nervous system tumors.
Thirty-four patients (34%) had grade II mucositis, which included the largest number of cases of mucositis caused by chemotherapy. The lowest number of patients with mucositis caused by chemotherapy was observed in grade IV disease (13 patients), and grade II mucositis was the most common (Table 2). Although there was no statistically significant relationship between the severity of mucositis and the gender of patients (P-value = 0.115), the number of male patients was four times that of female patients in grade III mucositis (Table 2).
| Variable | Grade I | Grade II | Grade III | Grade IV | Total |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 17 | 18 | 20 | 6 | 61 |
| Female | 11 | 16 | 5 | 7 | 39 |
| Total | 28 | 34 | 25 | 13 | 100 |
In this study, patients were included in three age ranges: Zero to five years, 5 - 10 years, and 10 - 16 years. The second age group had the highest number of mucositis patients, and the third age group had the lowest number. Based on the chi-square statistical test, there was no significant relationship between age and severity of mucositis in patients (P-value = 0.543) (Table 3).
| Variable | Grade I | Grade II | Grade III | Grade IV | Total |
|---|---|---|---|---|---|
| Age group (y) | |||||
| 0 - 5 | 10 | 11 | 11 | 2 | 34 |
| 6 - 10 | 9 | 16 | 8 | 7 | 40 |
| 11 - 16 | 9 | 7 | 6 | 4 | 26 |
| Total | 28 | 34 | 25 | 13 | 100 |
The highest number of patients with mucositis was observed in ALL malignancy (64 patients), with 26 patients in the second age group (6 - 10 years), 23 patients in the third age group (11 - 16 years), and 14 patients in the first age group (0 - 5 years) (Table 4).
| Age Ranges | Mucositis | Total | ||||
|---|---|---|---|---|---|---|
| Grade I | Grade II | Grade III | Grade IV | |||
| AML | 12 | |||||
| Second | 1 | 6 | 4 | - | 11 | |
| Third | 1 | 0 | 0 | - | 1 | |
| ALL | 64 | |||||
| First | 7 | 0 | 6 | 1 | 14 | |
| Second | 7 | 9 | 4 | 6 | 26 | |
| Third | 8 | 7 | 5 | 3 | 23 | |
| Other tumors | 24 | |||||
| First | 3 | 10 | 5 | 1 | 19 | |
| Second | 1 | 1 | 0 | 1 | 3 | |
| Third | 0 | 0 | 1 | 1 | 2 | |
Abbreviations: AML, acute myeloblastic leukemia; ALL, acute lymphoblastic leukemia.
a First age group (0 - 5 years), second age group (6 - 10 years), third age group (11 - 16 years).
The lowest number of patients with mucositis caused by chemotherapy was observed in AML malignancy, with 12 patients. No significant relationship was found between the types of mucositis caused by chemotherapy and the type of malignancy (P-value = 0.252).
Although chemotherapy protocols included several drugs, high-dose methotrexate, high-dose cytarabine, and anthracyclines were more likely to cause mucositis than other drugs. Mucositis following radiotherapy occurred only in three patients with nasopharyngeal rhabdomyosarcoma and medulloblastoma who underwent head and neck radiotherapy.
5. Discussion
In the present study, the highest number of patients with mucositis caused by chemotherapy had grade II mucositis, while the least number of patients had grade IV mucositis. The second age group (6 - 10 years) had the highest number of mucositis patients, and the third age group (11 - 16 years) had the lowest number. There was no significant relationship between age and the severity of mucositis in patients. Although the relationship between the severity of mucositis and the gender of patients did not show a statistically significant difference, in grade III mucositis, the number of male patients was four times that of female patients.
In the study by Allen et al., which aimed to investigate the prevalence and risk factors of oral mucositis in patients hospitalized in the oncology department, it was determined that the WHO Index of oral mucositis was 32.6% grade I, 34.9% grade II, 14.0% grade III, and 18.6% grade IV. These findings are consistent with the present study, where the highest percentage of mucositis was observed in grade II (9).
The findings of another study indicated that, unlike adults, in children with cancer undergoing chemotherapy, the incidence of oral mucositis is very low. However, the present study focused exclusively on children (10). The differences observed between the above studies could be attributed to variations in the type of cancer and the treatment regimens used.
The study by Sonis and Clark showed that the prevalence of mucositis in children is higher than in adults with the same malignancy (11). In the current study, the age range of the patients was between 1.3 and 16 years, whereas the Sonis study compared children with adult patients.
The higher occurrence of mucositis in younger patients may be attributed to the higher rate of mitosis in basal cells. This increased mitotic activity can cause the mucous epithelium to lose its ability to regenerate itself, leading to atrophy, thinning, and ulceration of the tissue.
The study by Cheng et al. examined the risk factors of oral mucositis in children and adults undergoing chemotherapy and found no significant relationship between oral mucositis and age (12).
Similar to the results obtained in the present study, some studies have rejected the existence of a relationship between the gender of patients and the occurrence of oral mucositis. However, Gebri et al. reported female sex as an independent risk factor for the occurrence of oral mucositis (13). In contrast, a study by Atinna et al. found the prevalence of mucositis to be 50%, with no significant statistical difference in terms of gender, although a higher prevalence was observed in patients over 10 years old (3).
Given the differing results across studies, the effect of age and gender on the incidence and severity of oral mucositis in patients undergoing chemotherapy remains unclear, emphasizing the necessity for further research on the impact of these factors.
In the present study, the highest number of patients with mucositis was observed in ALL, followed by AML. This finding aligns with the study by Dehabadi et al., in which most patients participating had ALL, and the majority of patients with oral mucositis belonged to this group (14).
In the first three months of treatment, a significant number of patients with ALL, more than one-third of neuroblastoma patients, and over one-fifth of rhabdomyosarcoma and osteosarcoma patients developed oral mucositis. This incidence increased significantly in the second three months, during which all patients with rhabdomyosarcoma, neuroblastoma, and osteosarcoma, as well as most patients with ALL, experienced oral mucositis.
While some studies argue that mucositis is not directly related to a specific malignancy, others suggest that certain types of cancer may exacerbate the severity of oral mucositis. For instance, the study by Guimaraes et al. demonstrated that patients with hematological malignancies are at a higher risk of developing severe oral mucositis compared to those with solid tumors (15).
5.1. Conclusions
The findings of the present study showed that the highest number of mucositis patients (78%) occurred in ALL and AML. The highest number of patients with mucositis was observed in grade II disease, while the lowest number of patients with mucositis caused by chemotherapy was observed in grade IV disease.
There was no significant relationship between the severity of mucositis and the gender or age of the patients. Additionally, no correlation was found between the severity of mucositis and the type of malignancy.