1. Background
Antibiotic resistance is one of the most pressing and critical challenges in medicine. The transfer of antibiotic-resistance genes has become widespread among various species of pathogenic bacteria that cause infectious diseases. Antibiotics exhibit different resistance mechanisms (1). The overuse of antibiotics has exacerbated the antibiotic resistance crisis (2). Given the growing resistance of pathogenic bacteria, it is crucial to investigate new compounds to combat this issue.
Biosurfactants are surface-active amphipathic molecules that have garnered attention for their bioactive properties and diverse structures (3). Studies reveal the antimicrobial roles of biosurfactants in relation to human health. Research confirms their antibacterial, antiviral, and antifungal properties, highlighting their potential as medicinal and therapeutic agents (4). Most biosurfactants are secondary metabolites, and their high potential in medical applications underscores their importance. They may serve as substitutes for conventional drugs and antimicrobial agents, significantly reducing hospital infections. Biosurfactants include various types such as lipopeptides, glycolipids, glycoproteins, and glycolipoproteins. Among those with notable antimicrobial effects are Surfactin and Fengicin (5). Specifically, glycolipid biosurfactants produced by Shewanella alga 12B and Bacillus pumilusSG have demonstrated antimicrobial properties and have been extensively studied (6).
Plant-derived compounds with biological activities, including antioxidant and antimicrobial properties, have gained attention for their role in combating infectious agents (7). Medicinal plants are known to boost the immune system, accelerating recovery. The chemicals in herbal extracts generally have minimal side effects, making them a reliable alternative. Beyond their antimicrobial properties, many of these plants exhibit pain-relieving effects (8). The active components in plant extracts, such as carotenoids and polyphenols, contribute to their antimicrobial and antioxidant activities (9). Phenolic compounds, synthesized through the phenylpropanoid pathway, disrupt bacterial membranes (10). Thyme extract, in particular, has demonstrated antimicrobial effects against foodborne pathogenic bacteria (11), inhibiting the growth of bacteria like Enterococcusfaecalis (12). It has also shown efficacy against pathogens such as EscherichiacoliO157:H7 (13).
2. Objectives
In this study, the synergistic effect of thyme extract combined with biosurfactants 12B and SG was investigated to assess their combined impact on pathogenic bacteria, both in planktonic and biofilm states. The synergistic effect of these antimicrobial compounds can be further enhanced when used with antibiotics, offering significant potential for treating infectious diseases. This is particularly important in cases of antibiotic resistance, as it provides a controlled process to reduce the resistance of pathogenic bacteria and effectively treat infected patients.
3. Methods
3.1. Sample Collection and Storage
The bacteria studied in this research were obtained from the Medical Sciences Department of Medical Bacteriology at Kerman University. To preserve the bacteria, a 20% glycerol stock was prepared for all bacterial strains. Biosurfactants 12B and SG were utilized as part of the study (6). Thyme, identified with the code SBUK 12456, was used to prepare the herbal extracts.
3.2. Preparation of Herbal Extract
Ten grams of thyme powder was mixed with 100 mL of methanol as a solvent, yielding a concentration equivalent to 10%. The mixture was then shaken in a shaker with methanol for 2 hours. After this period, the methanolic extract was filtered through Whatman filter paper and poured into a plate. The plate was placed in an oven at 37°C until the extract was completely dried. The final dried powder was combined with dimethyl sulfoxide (DMSO) solvent in the appropriate ratio to ensure complete dissolution (14).
3.3. Investigating Microbial Sensitivity by Disk Diffusion and Well Diffusion Methods
The disk diffusion and well diffusion methods were employed to investigate the antimicrobial effects of the combination of plant extract and biosurfactants. In these methods, a microbial suspension with a turbidity standard equivalent to half McFarland was prepared and cultured onto a nutrient agar medium using a sterile swab.
For the disk diffusion test, blank disks were immersed in solutions containing the combination of plant extract and biosurfactants at specific concentrations for 1 hour to allow thorough absorption. Dimethyl sulfoxide was used as the solvent for all extracts. The disks were then placed on a sterile plate to air dry slightly. Using sterile forceps, the disks were positioned on the nutrient agar medium where bacteria had been cultured. The plates were incubated at 37°C for 24 hours. After incubation, the zones of inhibition around the disks were measured. Cefazolin was used as a positive control.
For the well diffusion method, the preparation of microbial suspensions and extracts followed the same protocol as the disk diffusion test. Subsequently, sterile punches were used to create wells in the culture medium of each plate. A volume of 100 microliters of the plant extract and biosurfactant combination at a specific concentration was dispensed into each well. The plates were then incubated at 37°C for 24 hours (15).
3.4. Determination of Minimum Inhibitory Concentration
To determine the minimum inhibitory concentration (MIC) of the plant extract-biosurfactant combination, the serial dilution method was employed using a microtiter plate. Pathogenic bacteria were inoculated into nutrient broth culture and allowed to grow until they reached a turbidity equivalent to the standard turbidity of half McFarland. The bacterial suspension was then added to wells containing the antimicrobial compounds and incubated at 37°C. After 24 hours, the MIC value—the lowest concentration of the combination that inhibited visible bacterial growth—was measured and determined by reading the absorbance at a wavelength of 630 nm (16).
3.5. Determination of Minimum Bactericidal Concentration
To determine the minimum bactericidal concentration (MBC), the contents from the wells of the microplate with MIC concentrations and subsequent wells showing no turbidity were removed and cultured on nutrient agar plates. The plates were incubated at 37°C for 24 hours, after which they were examined for colony formation. The lowest concentration of the antimicrobial compounds that completely inhibited bacterial colony formation was recorded as the minimum bactericidal concentration for the target pathogenic bacteria (17, 18).
3.6. Determination of Fractional Inhibitory Concentration and Fractional Inhibitory Concentration Index
The fractional inhibitory concentration (FIC) was calculated individually for each antimicrobial substance. To evaluate the synergistic effects of their combination, the Fractional Inhibitory Concentration Index (FICI) was determined (19).
3.7. Investigating the Inhibitory Effect of Plant Extract-Biosurfactant Combination on Biofilm Formation
Microplates were used to evaluate the effect of the thyme extract–biosurfactant combination on biofilm formation inhibition. Each bacterium was inoculated into nutrient broth medium with antimicrobial compounds at concentrations corresponding to its MIC. Super-MIC and Sub-MIC concentrations of the bacteria were also inoculated in the wells. After 24 hours of incubation, the biofilm formation test was read as follows: The samples were washed with 0.1 M phosphate buffer and fixed using methanol for 10 minutes. They were then stained with 1% crystal violet for 30 minutes, washed three times with distilled water, and treated with 33% acetic acid. Finally, the biofilm samples were analyzed at a wavelength of 490 nm using an ELISA reader (BioTek Instruments, Assay: Quick Read), and the results of the thyme extract–biosurfactant combination's effect on biofilm formation inhibition were determined (20).
3.8. Investigating the Effect of Plant Extract-Biosurfactant Combination on the Destruction of Formed Biofilms
For this test, a microplate was used, and for each bacterium, wells were prepared with concentrations at the MIC, Super MIC, and Sub MIC. Bacteria were inoculated into a nutrient broth medium. After 24 hours of incubation, the thyme extract–biosurfactant combination was added to the wells, and the plates were incubated for another 24 hours. After this incubation period, the samples were removed and washed with 0.1 M phosphate buffer. They were then fixed using methanol for 10 minutes and stained with 1% crystal violet. After 30 minutes, the wells were washed three times with distilled water. Finally, 33% acetic acid was added to the wells, and the biofilm samples were analyzed at a wavelength of 490 nm using an ELISA reader. The results of the effect of the thyme extract–biosurfactant combination on biofilm destruction were determined (21).
3.9. Investigating the Effect of Plant Extract-Biosurfactant Combination on Dehydrogenase Enzyme Activity of Biofilm Bacterial Cells
This test was conducted to measure the viability of bacteria isolated from biofilms treated with various dilutions of the plant extract–biosurfactant combination, using TTC dye. A higher pigment production indicates greater dehydrogenase enzyme activity and a higher number of viable cells in the culture medium. To investigate dehydrogenase enzyme activity, microbial biofilms were first formed, followed by the addition of antimicrobial compounds at specific concentrations to the biofilms. After 24 hours of incubation, 50 µL of TTC dye was added to both treatment and control wells, and the plates were incubated at 37°C for 3 hours. Following the incubation period, the optical absorption of the wells was measured at a wavelength of 590 nm using an ELISA reader (22).
3.10. Statistical Analysis of Findings
In this research, each test was performed three times. The obtained data were analyzed using SPSS software version 26 and the ANOVA test. Each studied parameter was evaluated using LSD tests at a significance level of 0.05.
4. Results
4.1. Investigating Microbial Sensitivity by Disk Diffusion and Well Diffusion Methods
The average diameter of the inhibition zones for the combination of thyme extract and biosurfactants against four pathogenic bacteria, as determined by the disk diffusion and well diffusion methods, is presented in Table 1.
| Pathogenic Bacteria | Zone of Inhibition of Thyme-SG Combination (mm), Disk Diffusion | Zone of Inhibition of Thyme-12B Combination (mm), Disk Diffusion | Zone of Inhibition of Thyme-SG Combination (mm), Well Diffusion | Zone of Inhibition of Thyme-12B Combination (mm), Well Diffusion |
|---|---|---|---|---|
| MRSA | 16 | 15 | 16 | 16 |
| Escherichia coli | 19 | 16 | 16 | 16 |
| Acinetobacter baumannii | 17 | 15 | 17 | 16 |
| Bacillus cereus | 15 | 15 | 17 | 16 |
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
4.2. Minimum Inhibitory Concentration Results of Antimicrobial Compounds on Target Pathogenic Bacteria
The MIC results of the antimicrobial compounds on the target bacteria are presented in Table 2.
| MIC (mg/mL) | MRSA | Escherichia coli | Acinetobacter baumannii | Bacillus cereus |
|---|---|---|---|---|
| Thyme-SG | 6.25 | 3.125 | 12.5 | 12.5 |
| Thyme-12B | 25 | 3.125 | 12.5 | 25 |
Abbreviations: MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus.
4.3. Minimum Bactericidal Concentration Results of Antimicrobial Compounds on Target Pathogenic Bacteria
The MBC results of the antimicrobial compounds on the target bacteria are presented in Table 3.
| MBC (mg/mL) | MRSA | Escherichia coli | Acinetobacter baumannii | Bacillus cereus |
|---|---|---|---|---|
| Thyme-SG | 12.5 | 6.25 | 25 | 25 |
| Thyme-12B | 50 | 6.25 | 25 | 50 |
Abbreviations: MBC, minimum bactericidal concentration; MRSA, methicillin-resistant Staphylococcus aureus.
4.4. Results of Fractional Inhibitory Concentration and Fractional Inhibitory Concentration Index of Antimicrobial Compounds on Target Pathogenic Bacteria
The FIC value was determined for the thyme extract and biosurfactants individually, and the FICI for the combination of thyme extract and biosurfactants was calculated. The results are presented in Table 4.
| FICI | MRSA | Escherichia coli | Acinetobacter baumannii | Bacillus cereus |
|---|---|---|---|---|
| Thyme-SG | 1.25 | 0.5 | 1.5 | 1.5 |
| Thyme-12B | 6 | 0.5 | 3 | 2 |
Abbreviations: FICI, fractional inhibitory concentration index; MRSA, methicillin-resistant Staphylococcusaureus.
Based on the FICI results, the combination of thyme extract with SG and 12B biosurfactants exhibited a synergistic effect against E. coli but showed no significant effect on methicillin-resistant Staphylococcusaureus (MRSA), A. baumannii, and B. cereus bacteria.
4.5. Investigating the Inhibition of Biofilm Formation by Thyme Extract in Combination with SG and 12B Biosurfactants at Different Concentrations on Target Pathogenic Bacteria
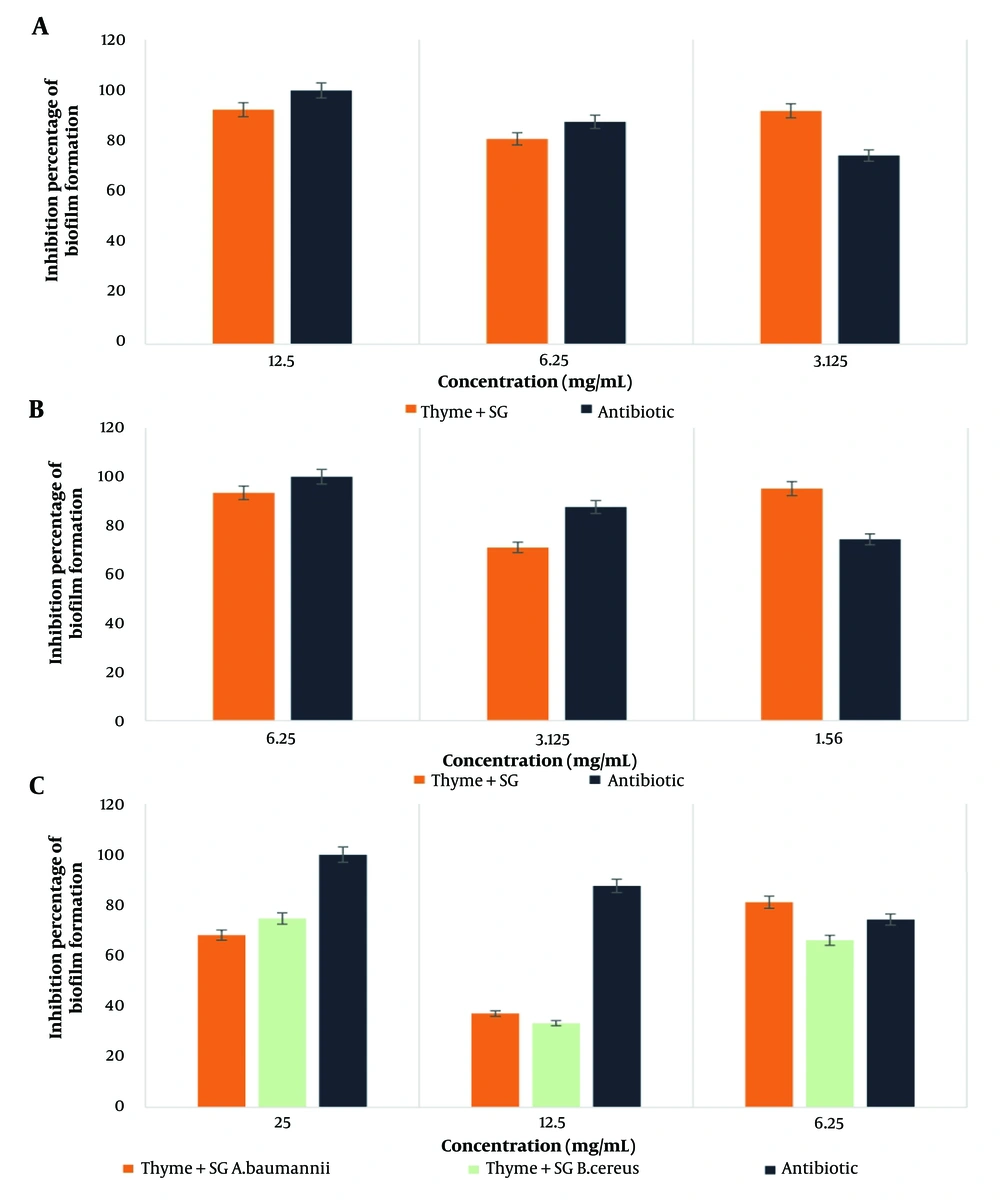
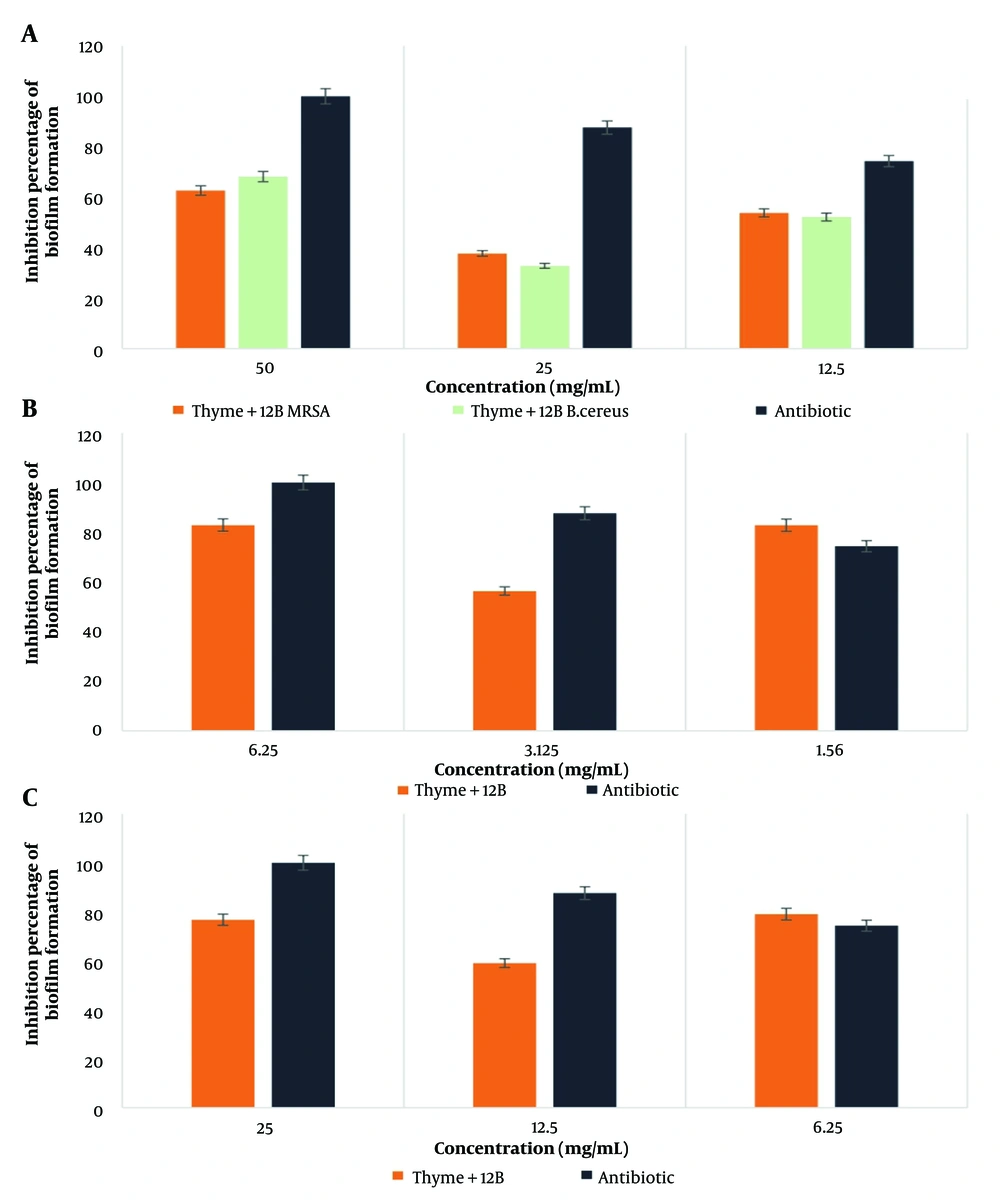
The highest percentage of biofilm formation inhibition by the thyme-SG combination was observed against E. coli bacteria at a concentration of 1.56 mg/mL. Similarly, for the thyme-12B combination, the highest inhibition was noted against E. coli bacteria at a concentration of 6.25 mg/mL. According to the statistical results, the effect of the thyme-biosurfactant combinations (SG and 12B) showed a significant difference only in the case of MRSA bacteria at the 0.05 significance level. The results are illustrated in Figures 1 and 2 (Cefazolin was used as a control).
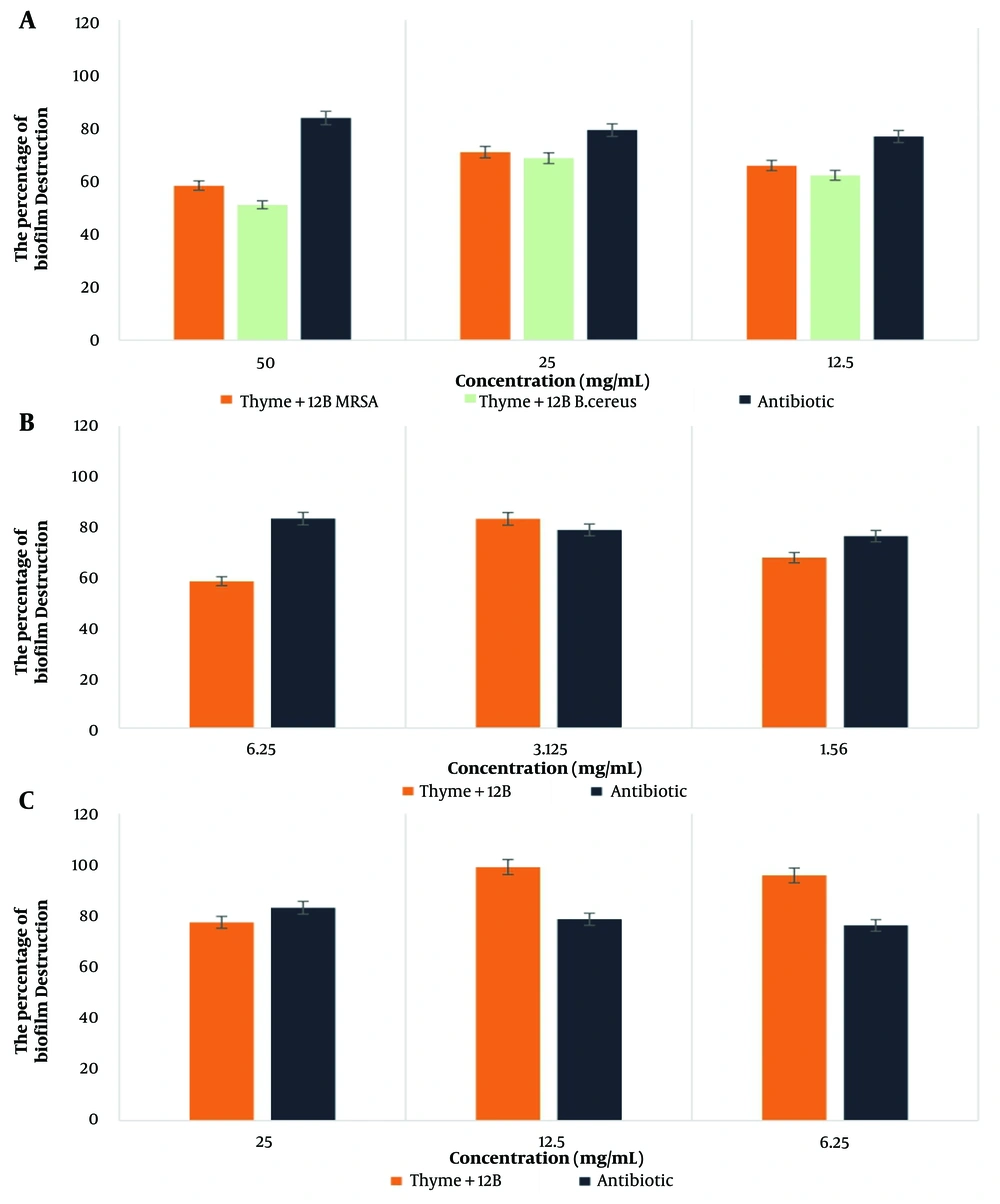
4.6. Investigating Biofilm Destruction by Thyme Extract in Combination with SG and 12B Biosurfactants in Different Concentrations on target Pathogenic Bacteria
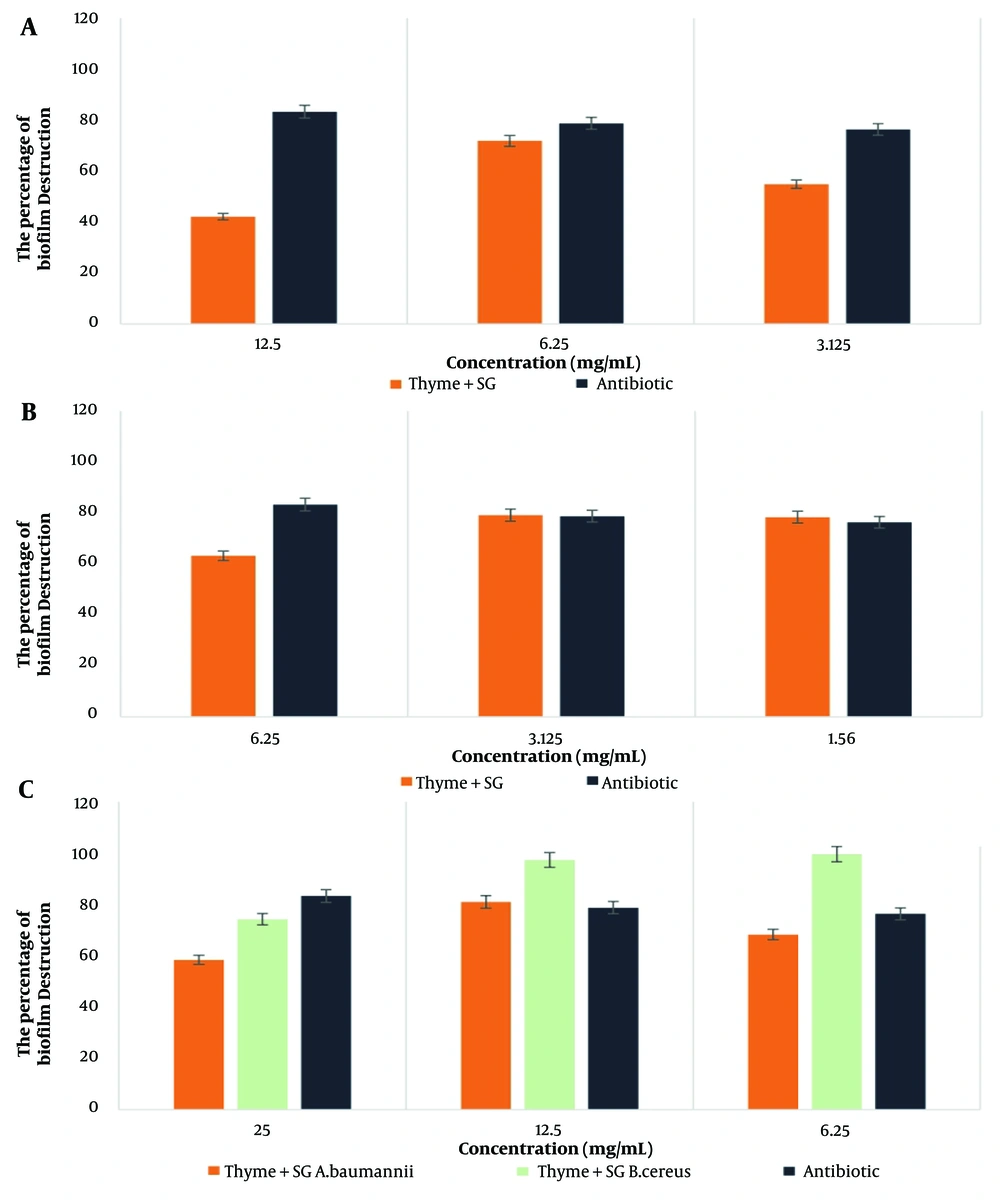
The highest percentage of biofilm destruction by the thyme-SG combination was observed against B. cereus bacteria at a concentration of 6.25 mg/mL, while for the thyme-12B combination, the highest effect was noted against A. baumannii bacteria at a concentration of 12.5 mg/mL. According to the statistical results, the combination of thyme with SG and 12B biosurfactants showed a significant difference only in the case of B. cereus bacteria at the 0.05 significance level. The results are presented in Figures 3 and 4.
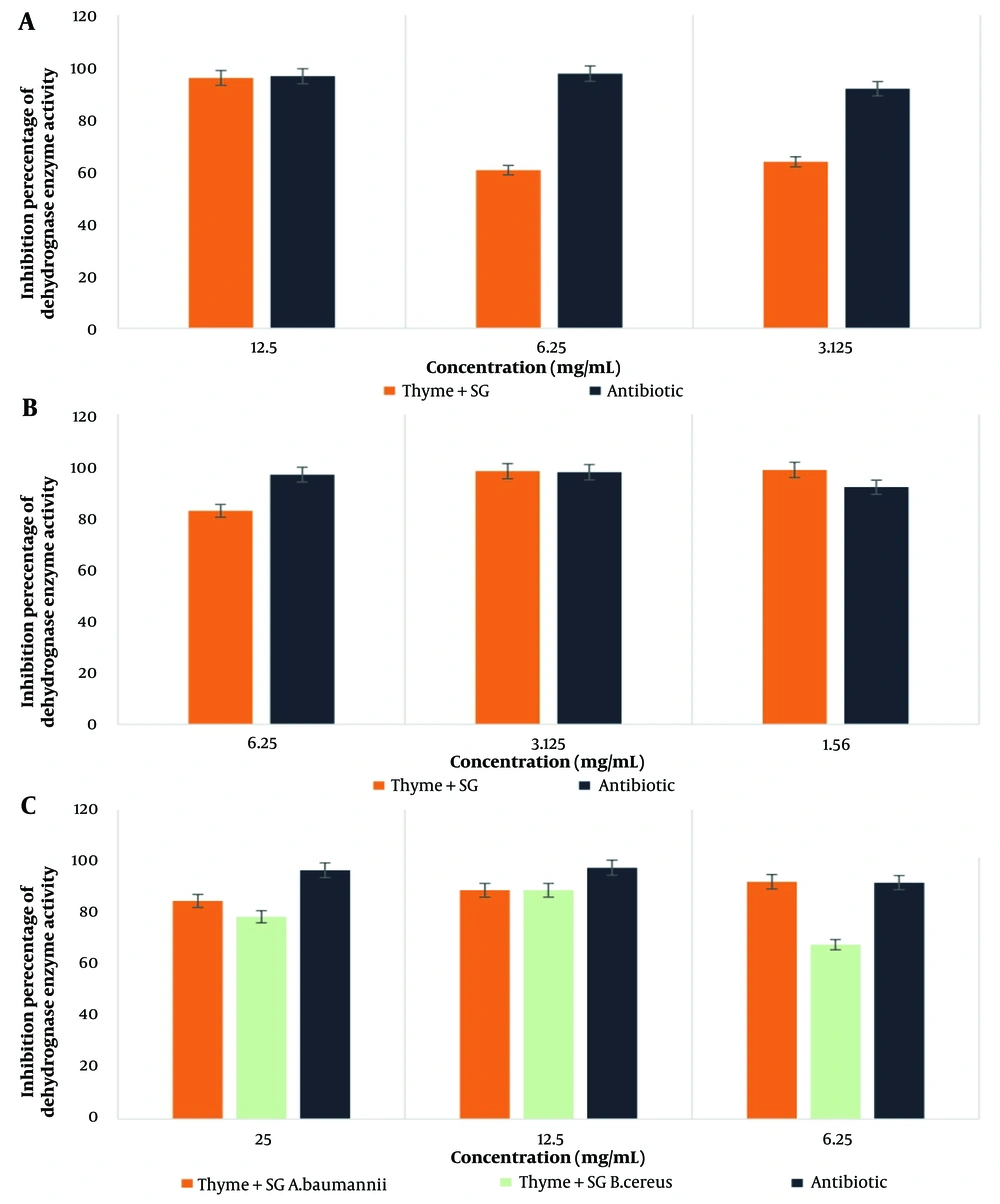
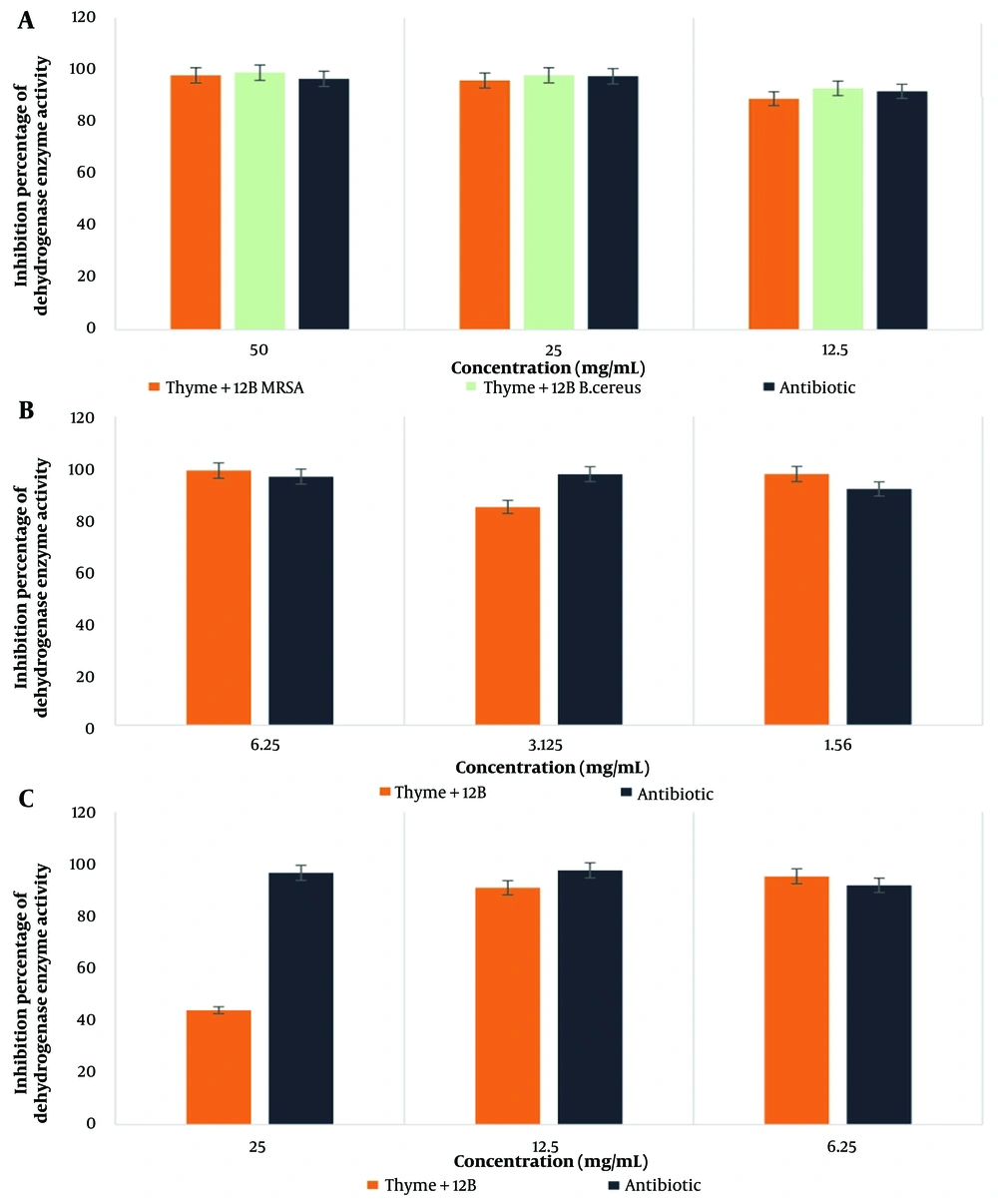
4.7. Investigating the Activity of Bacterial Dehydrogenase Enzyme by Thyme Extract in Combination with Biosurfactants SG and 12B in Different Concentrations on the Target Pathogenic Bacteria
The highest percentage of dehydrogenase enzyme activity for the thyme-SG combination was observed in E. coli bacteria at a concentration of 1.56 mg/mL, while for the thyme-12B combination, the highest activity was also noted in E. coli bacteria at a concentration of 6.25 mg/ml. According to the statistical results, the effect of thyme in combination with SG and 12B biosurfactants showed a significant difference only in the case of MRSA bacteria at the 0.05 significance level. The results are presented in Figures 5 and 6.
5. Discussion
Medicinal plants are effective in treating infectious diseases and hold promise as sources of new antimicrobial agents. Some phytochemicals exhibit intrinsic antibacterial activity as well as resistance-modifying properties (23). According to the research conducted by Mogana et al. in 2020, resistance to plant extracts is more prevalent in gram-negative bacteria than in gram-positive bacteria. The lipophilic or hydrophilic nature of the compounds also influences their activity against microorganisms. Compounds that are more effective against gram-negative bacteria tend to be less lipophilic, likely due to the structure of gram-negative bacterial cell walls, which contain higher lipid content (17).
In this study, methanolic extract of thyme and biosurfactants were prepared, demonstrating evident antimicrobial activity. A 2019 study by Khameneh et al. reported that the concurrent use of plant extracts with antibiotics reduced the MIC values of antibiotics, leading to observed synergistic effects (24).
For the combination of thyme extract and biosurfactants in the disk diffusion method, E. coli exhibited the largest zone of inhibition: 19 mm with the thyme-SG combination and 16 mm with thyme-12B. The combination of thyme with SG and 12B biosurfactants showed a synergistic effect against E. coli bacteria. Biosurfactants are natural molecules with antimicrobial and anti-biofilm properties due to their amphiphilic nature. Their antimicrobial action stems from their ability to disrupt membrane integrity, leading to cell lysis and metabolite leakage. Additionally, alterations in membrane structure and disruptions in protein configurations affect critical membrane functions, including transport and energy production.
Beyond their antimicrobial properties, the anti-biofilm activity of biosurfactants relates to their ability to influence bacterial adhesion and migration on surfaces by modifying surface tension and the charge of bacterial cell walls. Furthermore, they impact the expression of biofilm-related genes, enhancing their effectiveness against biofilm-forming pathogens (25, 26).
The combination of thyme-SG exhibited the highest effect in inhibiting biofilm formation on E. coli bacteria, while it had the lowest impact on B. cereus. Similarly, the combination of thyme-12B demonstrated a higher impact on E. coli bacteria and the least effect on B. cereus, with a value of 32.78%. When comparing these results with those obtained from the control antibiotic, cefazolin, it becomes evident that these compounds have a significant impact on pathogenic bacteria, affecting both antibiotic-resistant and non-antibiotic-resistant strains.
For biofilm destruction, the combination of thyme-SG showed the greatest impact on B. cereus bacteria and the lowest on MRSA, with a value of 42.10%. Meanwhile, the combination of thyme-12B achieved the highest effect on A. baumannii bacteria and the least impact on B. cereus, with a value of 50.71%.
Among the tested combinations of plant extracts and biosurfactants, the combination of thyme-12B exhibited the most significant inhibitory effect on E. coli and B. cereus bacteria, achieving a value of 98.97% inhibition on dehydrogenase enzyme activity.
5.1. Conclusions
The findings of this research demonstrated the acceptable efficiency of the combination of thyme extract with SG and 12B biosurfactants. This combination shows potential as an antimicrobial and anti-biofilm agent and could be effectively used alongside antibiotics in the treatment of microbial infections. The combination of thyme extract with biosurfactants represents a novel and promising approach in the field of medicine. However, to advance the application of these antimicrobial and anti-biofilm compounds, further studies and research are necessary to evaluate their effects in vivo and to measure their efficacy under clinical conditions.