1. Background
Periodontal diseases, including gingivitis and periodontitis, are significant public health concerns due to their high prevalence and the potential to cause severe oral and systemic health complications (1). Gingivitis is marked by gum inflammation, whereas periodontitis involves the destruction of tooth-supporting structures such as the periodontal ligament and alveolar bone, potentially leading to tooth loss if untreated (2). These conditions arise from a complex interaction between pathogenic bacteria in dental plaque and the host immune response, resulting in chronic inflammation (3). Beyond their oral health implications, periodontal diseases have been linked to systemic conditions, including cardiovascular diseases, diabetes, and adverse pregnancy outcomes, underscoring their broader public health impact (4, 5).
Globally, approximately 45 - 50% of adults are affected by periodontal diseases, with severe periodontitis affecting around 11% of individuals, ranking it as the sixth most prevalent condition worldwide (6, 7). In Iran, moderate-to-severe periodontal diseases are reported in 35 - 50% of the population, emphasizing the need for targeted prevention and treatment strategies (8).
Genetic factors, such as blood group antigens, have been implicated in susceptibility to various diseases, including periodontal conditions (9). The ABO blood group system classifies individuals into groups A, B, AB, and O based on specific antigens on red blood cells, while the Rh factor categorizes individuals as Rh-positive or Rh-negative depending on the presence or absence of the Rh antigen (10). These genetic markers may influence periodontal health through several proposed mechanisms:
1.1. Immune Response Modulation
Blood group antigens can influence the host's immune system. Specific blood groups may affect levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which are critical in periodontitis pathogenesis (11).
1.2. Microbial Adherence
Certain antigens may enhance the adherence of periodontal pathogens like Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans to oral tissues, increasing infection susceptibility (12).
1.3. Inflammatory Mediator Profiles
Variations in blood group antigens may regulate the production or activity of inflammatory mediators, exacerbating tissue destruction in periodontal diseases (13).
The Rh system may also contribute to periodontal disease susceptibility. Rh-positive individuals have been suggested to exhibit elevated levels of inflammatory cytokines compared to Rh-negative individuals, potentially intensifying the inflammatory response in periodontal tissues (14). However, these mechanisms remain speculative and require further investigation to determine their clinical significance.
Previous studies have produced conflicting findings regarding the relationship between ABO blood groups, Rh factor, and periodontal diseases (15-17). While some research suggests that specific blood groups may predispose individuals to periodontal diseases through differences in immune response, bacterial adhesion, or inflammatory mediator profiles (9, 18-20), evidence on the role of the Rh factor is limited and inconsistent. These discrepancies underscore the need for further research to clarify these associations.
Understanding genetic predispositions such as ABO and Rh antigens provides valuable insights into the pathophysiology of periodontal diseases. Such knowledge could inform the development of personalized preventive and therapeutic strategies in dentistry.
2. Objectives
By examining the associations between these genetic factors and periodontal disease prevalence, this study aims to support the advancement of individualized dental care tailored to the genetic profiles of at-risk populations.
3. Methods
3.1. Study Design and Participants
This descriptive-analytical cross-sectional study was conducted in 2022 at the Blood Transfusion Organization in Saravan, Sistan and Baluchestan province, Iran. The sample size of 368 participants was determined through a power analysis to detect statistically significant differences in the prevalence of periodontal diseases among ABO blood groups and Rh systems. Assuming a moderate effect size (Cohen's w = 0.3), an alpha level of 0.05, and a power of 80%, the required sample size was calculated to be approximately 320. To account for potential data loss and to ensure adequate subgroup representation, particularly for the Rh-negative group, the final sample size was increased to 368 participants. This adjustment ensured sufficient power to compare periodontal disease prevalence across all ABO and Rh categories.
The 368 participants included 200 men and 168 women, aged between 19 and 57 years, with a mean age of 34.96 ± 8.99 years. Each ABO blood group (A, B, AB, O) had 92 participants.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria comprised individuals aged 18 years and older who had not undergone periodontal treatment in the past six months. Exclusion criteria included systemic diseases that could affect periodontal health (e.g., diabetes, immunocompromising conditions), pregnancy or breastfeeding, and the use of medications known to influence periodontal status (e.g., immunosuppressants, anticoagulants).
3.3. Periodontal Examination
Clinical periodontal examinations were conducted by a calibrated dentist to ensure consistency and reliability. The assessments included evaluations of gingival inflammation, periodontal pocket depth, clinical attachment loss, and bleeding on probing. Based on these examinations, participants were categorized into two groups according to their periodontal status: Periodontally healthy and periodontally diseased (comprising both gingivitis and periodontitis).
3.4. Blood Group Determination
Blood samples were obtained from each participant to identify their ABO and Rh blood groups using the standard hemagglutination method. Participants were then classified into one of the four blood groups (A, B, AB, or O) and further categorized as either Rh-positive or Rh-negative.
3.5. Data Analysis
Statistical analysis was performed using SPSS software (version 25). Quantitative variables, such as age, were summarized as means and standard deviations for normally distributed data, with the distribution assessed using the Shapiro-Wilk test. For normally distributed data, t-tests or ANOVA were used for comparisons between groups, as appropriate. Non-normally distributed data were analyzed using non-parametric tests, including the Mann-Whitney U test or Kruskal-Wallis test. As no continuous quantitative variables such as periodontal pocket depth or clinical attachment level were included in the analysis, the quantitative measures were limited to demographic variables like age.
The chi-squared test was applied to evaluate the association between blood groups, Rh factor, and the prevalence of periodontal diseases. Logistic regression analysis was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs), with a significance level of P < 0.05. The logistic regression model included adjustments for potential confounders such as age, gender, and smoking status to provide a precise assessment of the relationship between blood groups, Rh factor, and periodontal diseases.
4. Results
4.1. Demographic Characteristics
A total of 368 participants were included in the study, comprising 200 women (54.3%) and 168 men (45.7%). The participants' ages ranged from 19 to 57 years, with a mean age of 34.96 ± 8.99 years. The distribution of participants across ABO blood groups was equal, with 92 individuals in each group (Table 1).
| Variables | Frequency (%) |
|---|---|
| Gender | |
| Female | 200 (54.3) |
| Male | 168 (45.7) |
| RH system | |
| Positive | 312 (84.8) |
| Negative | 56 (15.2) |
| Periodontal diseases | |
| Present | 200 (54.3) |
| Absent | 168 (45.7) |
4.2. Prevalence of Periodontal Diseases in Each ABO Blood Group
Among the participants, 54.3% (n = 200) had periodontal diseases, while 45.6% (n = 168) were periodontally healthy. A statistically significant difference was observed in the prevalence of periodontal diseases among the different ABO blood groups (P < 0.001) (Table 2).
| Periodontal Diseases | Blood Groups | Total | P-Value | |||
|---|---|---|---|---|---|---|
| A | B | AB | O | |||
| Present | 46 (12.5) | 69 (18.75) | 31 (8.43) | 54 (14.68) | 200 (54.36) | < 0.001 |
| Absent | 46 (12.5) | 23 (6.25) | 61 (16.57) | 38 (10.32) | 168 (45.64) | |
| Total | 92 (25) | 92 (25) | 92 (25) | 92 (25) | 368 (100) | |
a Values are expressed as No. (%).
4.3. Prevalence of Periodontal Diseases in Each Rh System
Rh-positive individuals constituted 84.78% of the sample, while 15.21% were Rh-negative. The prevalence of periodontal diseases was 51.08% in the Rh-positive group and 3.26% in the Rh-negative group, with this difference being statistically significant (P < 0.001) (Table 3).
| Periodontal Diseases | Rh System | Total | P-Value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Present | 188 (51.08) | 12 (3.26) | 200 (54.36) | < 0.001 |
| Absent | 124 (33.69) | 44 (11.95) | 168 (45.64) | |
| Total | 312 (84.78) | 56 (15.21) | 368 (100) | |
a Values are expressed as No. (%).
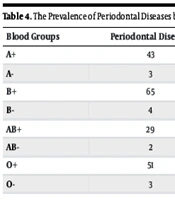
4.4. Detailed Prevalence in Each ABO and Rh Subgroup
The prevalence of periodontal diseases was further analyzed by stratifying each blood group into Rh-positive and Rh-negative subgroups. The comparison revealed significant variability in disease prevalence across these subgroups (Table 4).
| Blood Groups | Periodontal Diseases | Healthy Cases | Prevalence in Subgroup (%) | Prevalence in Total Sample (%) |
|---|---|---|---|---|
| A+ | 43 | 35 | 55.1 | 11.7 |
| A- | 3 | 11 | 21.4 | 0.8 |
| B+ | 65 | 13 | 83.3 | 17.7 |
| B- | 4 | 10 | 28.6 | 1.1 |
| AB+ | 29 | 49 | 37.2 | 7.9 |
| AB- | 2 | 12 | 14.3 | 0.5 |
| O+ | 51 | 27 | 65.4 | 13.9 |
| O- | 3 | 11 | 21.4 | 0.8 |
4.5. Logistic Regression Analysis of ABO Blood Groups and Rh System
Logistic regression analysis was performed to assess the relationship between blood types and periodontal diseases. The results indicated that individuals with blood group B were significantly more likely to develop periodontal diseases compared to those with blood group O (OR: 2.111; p = 0.020). Conversely, individuals with blood group AB exhibited a significantly lower likelihood of periodontal diseases (OR: 0.358; P = 0.001). No significant association was found for blood group A (Table 5).
| Blood Groups | Odds Ratio (95% Confidence Intervals) | P-Value |
|---|---|---|
| O | 1 | - |
| A | 0.704 (0.393 - 1.260) | 0.237 |
| B | 2.111 (1.126 - 3.958) | 0.020 |
| AB | 0.358 (0.196 - 0.651) | 0.001 |
The findings suggest that blood type is an influential factor in the development of periodontal diseases. According to the logistic regression results presented in Table 5, individuals with blood type B have a higher likelihood of developing periodontal diseases compared to those with blood type O, while the likelihood is lower in individuals with blood types A and AB.
Additionally, further analysis revealed that Rh-positive individuals are significantly more likely to develop periodontal diseases compared to Rh-negative individuals (OR: 5.559; P < 0.001) (Table 6).
| Rh System | Odds Ratio (95% Confidence Intervals) | P-Value |
|---|---|---|
| Positive | 5.559 (2.824 - 10.944) | < 0.001 |
| Negative | 1 | - |
5. Discussion
This study underscores significant associations between ABO blood groups, Rh status, and the prevalence of periodontal diseases. Individuals with blood group B had more than twice the odds of developing periodontal diseases compared to those with blood group O. Furthermore, Rh-positive individuals exhibited a more than fivefold increase in the likelihood of periodontal diseases compared to Rh-negative individuals. These findings emphasize the potential role of genetic factors, such as blood group antigens and Rh status, in influencing susceptibility to periodontal diseases.
5.1. Genetic Susceptibility and Pathophysiological Mechanisms
The differential susceptibility to periodontal diseases among ABO and Rh blood groups may be attributed to the roles of blood group antigens in immune function and inflammation. Blood group antigens are expressed on the surfaces of red blood cells and epithelial cells, including those lining the oral cavity, and they may influence microbial colonization and host immune responses. For instance, blood group B antigens might enhance the adhesion of pathogenic bacteria, such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, which are associated with periodontal diseases (11, 16). Conversely, individuals with blood group O lack A or B antigens, potentially limiting the range of bacterial interactions, which may account for their relatively lower susceptibility (13). The protective effect observed in blood group AB could result from a combined influence of both A and B antigens, potentially modulating immune responses to reduce susceptibility to inflammation-driven tissue damage (12).
The association with Rh-positive status likely arises from interactions between Rh antigens and immune components. Rh-positive individuals may exhibit increased levels of inflammatory cytokines, such as TNF-α and IL-6, which are critical mediators in periodontal tissue destruction (21). Additionally, Rh antigens might influence the regulation of vascular function and leukocyte activity, further amplifying the inflammatory response. The interplay between these genetic factors and local environmental triggers, such as microbial dysbiosis, may provide a mechanistic explanation for the observed increased risk in Rh-positive individuals (14).
5.2. Probability Comparisons
To further elucidate the relative risks, the prevalence of periodontal diseases was calculated for each blood group and Rh subgroup. Blood group B (Rh-positive) individuals exhibited the highest prevalence (23.72%), indicating a compounded risk from both genetic factors. Blood group AB (Rh-negative) showed the lowest prevalence (2.17%), suggesting a protective effect in this subgroup.
When comparing Rh-positive and Rh-negative individuals within each blood group, Rh-positive individuals consistently showed higher prevalence rates. For example, in blood group A, periodontal disease prevalence was 11.9% for Rh-positive individuals but only 0.54% for Rh-negative individuals. Similarly, in blood group O, the prevalence was 14.13% for Rh-positive individuals compared to 0.54% for Rh-negative individuals.
These differences highlight the additive nature of Rh-positive status as a risk factor across all blood groups. The compounded probability of periodontal diseases in individuals with blood group B and Rh-positive status underscores the importance of these genetic traits in determining disease susceptibility.
5.3. Comparison with Previous Studies
The association between blood group B and an elevated risk of periodontal diseases aligns with findings from prior research. For example, studies by Pai et al. and Jain et al. reported a higher prevalence of periodontal diseases among blood group B individuals compared to other blood groups (12, 21). These studies attributed the increased risk to genetic variations in inflammatory pathways and microbial interactions in blood group B individuals (12, 21). Our findings corroborate these conclusions, indicating that blood group B individuals are 2.111 times more likely to develop periodontal diseases compared to those with blood group O.
Our study also provides novel insights by highlighting the significant role of Rh-positive status as an independent risk factor, a factor not extensively explored in previous research. The strong association observed in Rh-positive individuals suggests that Rh antigens may interact with immune regulatory pathways, increasing susceptibility to inflammatory conditions like periodontitis (14, 16).
Studies from other regions, such as Europe and North America, have reported varying results. While some research has not found a significant association between blood groups and periodontal diseases, these discrepancies may stem from differences in genetic background, population-level antigen distributions, or environmental influences, such as diet and oral hygiene practices (11). For instance, the lower prevalence of blood group B in Western populations may reduce its overall impact on disease trends. Additionally, variations in healthcare access and cultural attitudes toward dental care could influence these associations (14).
Our findings support the hypothesis that genetic and environmental factors interact to shape disease susceptibility. While genetic predisposition plays a central role, environmental triggers such as poor oral hygiene, smoking, and systemic conditions like diabetes may act as modulators, either amplifying or mitigating the genetic risk (13).
5.4. Population Specificity and Generalizability
While these findings offer valuable insights, the study's generalizability is potentially limited by the homogeneity of the sample. The geographic and demographic characteristics of the participants may not represent other populations, particularly those with varying distributions of ABO and Rh antigens. Genetic diversity and environmental factors, such as oral hygiene practices, dietary habits, and access to dental care, could substantially influence the prevalence of periodontal diseases. Consequently, extending this research to include more diverse populations is essential for validating these findings.
5.5. Potential Biases and Limitations
Several limitations must be acknowledged when interpreting these findings. The cross-sectional design precludes the establishment of causal relationships, and the use of convenience sampling may introduce selection bias. Furthermore, confounding variables such as smoking, systemic conditions (e.g., diabetes), and socioeconomic status were not accounted for, which could influence the observed associations.
Specifically, behavioral factors such as smoking and oral hygiene practices, as well as systemic conditions like diabetes, are well-established influences on periodontal health and may interact with genetic predispositions. For example, smoking, a strong risk factor for periodontitis, may disproportionately affect individuals in certain genetic subgroups. The lack of adjustment for these variables in the present study highlights a critical limitation, underscoring the importance of future research incorporating detailed data on confounders to validate and refine these findings.
5.6. Clinical Implications
The identification of blood group B and Rh-positive status as significant risk factors for periodontal diseases has important implications for clinical practice. These findings suggest that dentists could incorporate ABO and Rh typing into risk assessment protocols. High-risk individuals, such as those with blood group B or Rh-positive status, may benefit from personalized preventive measures, including increased frequency of periodontal evaluations, enhanced oral hygiene education tailored to their risk profile, and early therapeutic interventions to prevent disease progression. Understanding the interplay between genetic susceptibility and periodontal disease may enable clinicians to develop targeted interventions that improve patient outcomes and reduce disease burden.
5.7. Future Directions
Future research should explore the mechanisms underlying the associations between ABO and Rh blood groups and periodontal diseases. Longitudinal studies are necessary to confirm causality and examine temporal dynamics. Expanding investigations to include additional genetic markers, such as single nucleotide polymorphisms (SNPs) linked to inflammatory pathways, could offer deeper insights. Additionally, studies focusing on the interaction between genetic predispositions and environmental or lifestyle factors, such as smoking and systemic health conditions, are warranted.
5.8. Conclusions
This study reveals a significant association between blood group B, Rh-positive status, and an increased prevalence of periodontal diseases, emphasizing the potential influence of genetic factors on disease susceptibility. These findings highlight the necessity for personalized preventive and therapeutic strategies in dental practice, particularly for individuals with blood group B and Rh-positive status. Further research is required to investigate the biological mechanisms underlying these associations and to design targeted interventions for individuals at elevated risk.