1. Background
Respiratory distress syndrome (RDS) is a common cause of neonatal intensive care unit (NICU) admission and mortality in preterm infants. The syndrome is primarily caused by a reduction in the production and secretion of surfactant. The greatest risk factor for RDS is prematurity. Additional risk factors include maternal diabetes, second-born twins, cesarean section delivery, and a history of RDS in siblings (1). Secondary surfactant deficiency may occur in infants with intrapartum asphyxia, pulmonary infections, pulmonary hemorrhage, and meconium aspiration syndrome (2-4). Clinical symptoms, which usually appear within minutes after birth, include tachypnea, intercostal and subcostal retractions, cyanosis, and grunting. Characteristic radiographic findings include a fine reticulogranular appearance in the lung parenchyma, along with an air bronchogram. Differential diagnoses include early-onset sepsis, pneumonia, cyanotic heart diseases, persistent pulmonary hypertension, aspiration syndromes, and transient tachypnea of the newborn.
The COVID-19 pandemic, caused by the novel SARS-CoV-2, resulted in over 7 million cases by 2023. According to recent data, this virus affects children of all ages, with higher severity in children under one year old (5, 6). Based on available epidemiological information, the incubation period of the disease usually varies from 3 to 7 days (7), and it is contagious during this period. Clinical manifestations include fever, cough, respiratory distress, shortness of breath, and pneumonia. In severe cases, neurological, digestive, liver, and respiratory complications may occur (8). Limited data is available regarding its effect on pregnancy outcomes because COVID-19 virus infection was not detected in humans before the pandemic. Pregnant women have increased susceptibility to respiratory pathogens and severe pneumonia due to physiological changes in the immune and cardiopulmonary systems, elevation of the diaphragm, increased oxygen consumption, and edema of the respiratory tract mucosa. Reports indicate that up to one in eight pregnant women may test positive for SARS-CoV-2 (9). Infection during pregnancy can potentially affect fetuses and neonates through various mechanisms: Increased rates of preterm birth (PTB), placental infection, and compromised gas and nutrient exchange, leading to intrauterine death or perinatal asphyxia; and through in utero, during delivery, or after birth transmission of the virus.
2. Objectives
There is limited information about RDS in preterm infants born to mothers infected with COVID-19. Distinguishing between COVID-19 disease with pulmonary involvement in infants born to infected mothers and cases of RDS is currently a challenge. This study was conducted to investigate the severity of RDS in premature infants with a gestational age of less than 34 weeks born to mothers infected with COVID-19.
3. Methods
This retrospective study was performed in a referral university hospital from March 2020 to September 2021. The inclusion criteria were preterm infants with a gestational age of 34 weeks or less who were admitted to the NICU, with defined maternal status concerning COVID-19 infection. Patients with major congenital anomalies, congenital heart disease other than patent ductus arteriosus (PDA) and atrial septal defect (ASD), genetic and chromosomal syndromes, severe birth asphyxia (apgar score less than 4 at the 5th minute), and lack of parental consent were excluded from this study. The study was approved by the Ethics Committee of Tabriz University of Medical Sciences with the code IR.TBZMED.REC.1400.1135. Written informed consent was obtained from parents. Based on a pilot study with a 25% difference between groups, with a power of 80% and alpha of 0.05, we estimated that 28 cases were needed for each group. Patients who met the inclusion criteria were randomly enrolled in two groups using a random number list generated by a random number generator in sequentially numbered, opaque, sealed, and stapled envelopes. This study included 30 premature infants with a gestational age of less than 34 weeks born to mothers with COVID-19 infection and admitted to the NICU at AL-Zahra Hospital (group P). Group N consisted of 30 hospitalized preterm infants born to mothers without clinical signs of COVID-19 infection and negative reverse transcription polymerase chain reaction (RT-PCR) tests for COVID-19 during the same study period. The diagnosis of COVID-19 infection in the pregnant women was confirmed by real-time RT-PCR tests of COVID-19 in throat swabs and lung involvement in CT scans.
The preterm infants born to infected mothers were admitted to an isolated room, and all staff wore masks, gloves, and gowns based on isolation protocols. PCR for COVID-19 in the nasopharyngeal swab test was conducted on the morning of the day following admission. We considered RT-PCR as the gold standard for the diagnosis of SARS-CoV-2. It is routine in our NICU to determine the severity of RDS using the ACORN respiratory score (Table 1).
| Score | 2 | 0 | 1 |
|---|---|---|---|
| Respiratory rate | > 80 | 40 - 60 | 60 - 80 |
| Oxygen | < 50 | No | ≤ 50% |
| Retraction | Severe | No | Mild to moderate |
| Grunting | Continuous (at rest) | No | With stimulation |
| Breathing sounds | Not audible | Audible | Decreased |
| Gestation age | < 30 | < 34 | 30 - 34 |
The ACORN is an algorithmic and sequential model of assessment, monitoring, intervention, and management of critically ill or at-risk infants. In this scoring system, the severity of respiratory distress is evaluated as follows: A score of less than five is considered mild, a score of 5 - 8 is moderate, and a score above eight is severe. Resuscitation in the delivery room was performed according to the neonatal resuscitation program (NRP). Stabilization of the preterm infant using nasal continuous positive airway pressure (NCPAP) in the delivery room by infant T-piece resuscitator (Fisher and Paykel Healthcare, Auckland, New Zealand) is our center's policy. Intubation was reserved for those who had apnea or ineffective respiration. After admission to the NICU, NCPAP was administered through intermittent short bilateral nasal prongs and nasal mask. Distending pressure was generated by a variable flow NCPAP device with a peak end-expiratory pressure (PEEP) of 6 - 7 cm H2O and a flow of 6 - 7 L/min (Fisher and Paykel Healthcare Limited, New Zealand). Surfactant was given to infants who met clinical and radiologic criteria for RDS by the INSURE method (intubation, surfactant administration, extubation to CPAP) within the first 6 hours of life. The targeted SpO2 was 90 - 94%. The primary outcome was the need for surfactant replacement therapy. The secondary outcomes were the duration of hospital stay, systemic signs of infection, and mortality. The data were analyzed using the IBM Statistical Package for Social Sciences (SPSS), version 22. An independent-samples t-test was employed to compare the means of quantitative variables. To compare proportions between two qualitative parameters, the chi-square test was utilized. The P < 0.05 values were considered statistically significant.
4. Results
The study population consisted of 27 (45%) boys and 33 (55%) girls. The mean gestational age and birth weight of the studied neonates were 30.8 ± 1.3 weeks and 1450 ± 205 grams, respectively. The demographic characteristics showed no statistically significant differences between the two groups (Table 2).
| Variables | Group P b (n = 30) | Group N c (n = 30) | P-Value |
|---|---|---|---|
| Gender (male/female) | 13/17 | 14/16 | 0.79 |
| Gestation age (wk) | 30.62 ± 2.48 | 31.24 ± 2.26 | 0.32 |
| Birth weight (g) | 1526 ± 424 | 1594 ± 411 | 0.53 |
| Maternal age (y) | 30.13 ± 6.47 | 30.72 ± 6.75 | 0.73 |
| Route of delivery CS | 27 (90) | 28 (93.3) | 0.64 |
| Antenatal corticosteroid therapy | 9 (30) | 4 (13.3) | 0.11 |
| Resuscitation after birth | 17 (56.7) | 6 (20) | 0.003 |
Abbreviation: CS, cesarean section.
a Values are expressed as No (%) or mean ± SD.
bGroup P: Preterm infants born to mothers with COVID- 19 infection.
c Group N: Preterm infants born to mothers without COVID-19 infection.
Maternal risk factors included hypertension (26.7%), preeclampsia (16.7%), and hypothyroidism (10%) in group P, and hypertension (16.7%), preeclampsia (10%), and hypothyroidism (10%) in group N, with a P-value of 0.58. Positive COVID-19 PCR was associated with positive chest CT scan findings in 17 (56.7%) mothers. Twenty-five (83.3%) neonates had positive COVID-19 PCR, of which 14 were born to mothers with negative chest CT scans, with a P-value of 0.51. The need for delivery room resuscitation (beyond initial steps) was detected in 17 (56.7%) neonates in group P and six (20%) neonates in group N, with a P-value of 0.003.
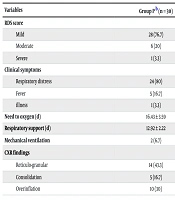
The clinical manifestations and respiratory management in both groups are shown in Table 3. Surfactant replacement therapy was needed in 21 (70%) patients in group N and 24 (80%) in group P, with a P-value of 0.39. C-reactive protein (CRP) was positive in 17 (56.6%) neonates in group P and six (20%) cases in group N, with a P-value of 0.003. Broad-spectrum antibiotics were used in 25 (83.3%) neonates in group P and 15 (50%) patients in group N, with a P-value of 0.02. Leukopenia was detected in 7 (23.3%) neonates in group N and 9 (30%) in group P, with a P-value of 0.57. Lymphopenia was detected in 14 (46.7%) in group N and 6 (20%) in group P, with a P-value of 0.09. Thrombocytopenia was seen in 4 (13.3%) in group N and 13 (21.7%) in group P, with a P-value of 0.21. The LDH levels greater than 1000 units/L were determined in 9 (30%) patients in group P. Mortality was observed in 2 (6.7%) patients in group P and 1 (3.3%) in group N, all of whom had gestational ages of less than 26 weeks, with a P-value of 0.55. Outcome data are presented in Table 4.
| Variables | Group P b (n = 30) | Group N c (n = 30) | P-Value |
|---|---|---|---|
| RDS score | 0.52 | ||
| Mild | 28 (76.7) | 26 (73.3) | |
| Moderate | 6 (20) | 8 (26.7) | |
| Severe | 1 (3.3) | 0 | |
| Clinical symptoms | 0.03 | ||
| Respiratory distress | 24 (80) | 30 (100) | |
| Fever | 5 (16.7) | 0 | |
| illness | 1 (3.3) | 0 | |
| Need to oxygen (d) | 16.43 ± 3.59 | 16.10 ± 3.59 | 0.94 |
| Respiratory support (d) | 12.92 ± 2.22 | 16.04 ± 3.72 | 0.47 |
| Mechanical ventilation | 2 (6.7) | 1 (3.3) | 0.55 |
| CXR findings | < 0.001 | ||
| Reticulo-granular | 14 (43.3) | 29 (96.7) | |
| Consolidation | 5 (16.7) | 0 | |
| Overinflation | 10 (30) | 1 (3.3) |
Abbreviations: RDS, respiratory distress syndrome; CXR, chest x-ray.
a Values are expressed as No (%) or mean ± SD.
b Group P: Preterm infants born to mothers with COVID- 19 infection.
c Group N: Preterm infants born to mothers without COVID-19 infection.
a Values are expressed as No (%) or mean ± SD.
b Group P: Preterm infants born to mothers with COVID- 19 infection.
c Group N: Preterm infants born to mothers without COVID-19 infection.
5. Discussion
The severity of RDS, the need for surfactant replacement therapy, the duration of hospital stay, and mortality were not significantly different in preterm infants born to mothers with and without COVID-19 infection in our study. However, delivery room resuscitation and signs and symptoms of systemic diseases were significantly more common in preterm infants born to mothers with COVID-19 infection. There is limited information about RDS in preterm infants of COVID-19 infected mothers. In one study, 122 unvaccinated pregnant women with COVID-19 infection, tested by RT-PCR nasopharyngeal swab, were enrolled. Mothers were asymptomatic in 60% of cases. Overall, the rate of PTB and NICU admission was 10.4%, with the majority being late preterm. Ten neonates were delivered at 32 - 37 weeks, two cases at 28 - 32 weeks, and one neonate at less than 28 weeks. The RDS was diagnosed in seven patients (5.6%). In contrast to our study, none of the neonates tested positive for COVID-19 infection (10). They reported a higher rate of RDS among newborns of mothers with severe COVID-19 symptoms. In a systematic review of 23 studies that reported on RDS among babies born to COVID-19-infected mothers, the total pooled prevalence of RDS was found to be 11.5% (95% CI: 7.4 - 17.3%). The meta-analysis evaluating the risk of RDS in neonates born to women with COVID-19 compared to those born to non-infected mothers revealed a pooled RR of 2.69 (95% CI: 1.77 to 4.17). High heterogeneity was observed, including variations in study design, population characteristics, and multiple countries, which may reflect differences in healthcare systems, resources, disease severity, and treatment protocols (11). The majority of the included studies in this meta-analysis did not provide sufficient detail on gestational age.
Newborns infected with COVID-19 had favorable outcomes in a few studies (12-17). The PCR-confirmed infected newborns can be asymptomatic or have mild to severe symptoms, including respiratory or gastrointestinal signs, apnea, difficulty breathing, cough, lethargy, poor feeding, feeding intolerance, and distended abdomen, mainly in premature babies (18-20). Symptomatic neonates generally recover in one to two weeks in most reports. However, long-term follow-up is currently lacking (21, 22). Man and coworkers studied 221 pregnant persons with COVID-19 and 227 COVID-19 exposed fetuses in a longitudinal cohort study. None of the infants in their study tested positive for SARS-CoV-2 at birth, and 17% (n = 34) were diagnosed with RDS (23). Fever, clinical signs and symptoms of sepsis, in addition to respiratory distress and positive CRP, were significantly more common in preterm infants born to mothers with COVID-19 in our study. This finding may be due to the lower gestational age in our studied preterm infants.
More than three-fourths of our cases had positive COVID-19 PCR results, in contrast to other studies. In the largest cohort study from China (24), none of the 86 infants had a positive result for COVID-19 via nasopharyngeal swabs. In a study in the United Kingdom, 12 of 244 neonates were positive for SARS-CoV-2 (25). Vertical transmission may occur during the antepartum, intrapartum, or postpartum period via the placenta, delivery canal, or direct contact due to breastfeeding after birth. The placenta and amniotic fluid were not examined for SARS-CoV-2 in our study. Therefore, no evidence is available that the virus was transmitted to the fetus during pregnancy or labor. The high rate of vertical transmission in our study may be related to breast milk feeding and the timing of PCR sampling within the first 48 hours after birth. It is estimated that 25% of neonates born to mothers infected with COVID-19 are admitted to neonatal care units (26). The standard for detecting COVID-19 infection is viral RNA detection using RT-PCR. However, this diagnostic method exhibits variable performance depending on sampling sources. Sensitivities for COVID-19 detection by RT-PCR test in nasal, bronchoalveolar lavage, feces, blood, and urine specimens were 63%, 93%, 29%, 1%, and 0%, respectively (27). The lack of detection of SARS-CoV-2 in the amniotic fluid may be due to its source of production from fetal urine. The mortality rate was 6.7% in preterm infants born to infected mothers in our study. At present, no specific treatments are available for COVID-19, and patients are symptomatically managed. Since the gestational age of expired infants was less than 27 weeks, it seems that poor neonatal outcomes are related to the severity of prematurity rather than neonatal COVID-19 infections. Adverse neonatal outcomes of infants of COVID-19 infected mothers, and death have been mainly attributed to prematurity or comorbidities. However, adverse perinatal outcomes such as stillbirth, intrauterine growth restriction, perinatal asphyxia, and severe neonatal pulmonary and systemic disease have been reported (12). Advanced delivery room resuscitation was significantly more common in neonates of COVID-19 infected mothers. The ACE receptors, which are the main receptors for the entry of SARS-CoV-2 into cells, have relative differences in newborn infants and are suggested to be a contributory factor in neonatal resistance to COVID-19 infection, but supporting evidence is not sufficient (28). Relative vitamin D deficiency in adults, increased comorbidities, and endothelial damage, along with chronic low-grade systemic inflammation with higher plasma levels of IL-6, TNF-α, and other innate cytokines, may be responsible for differences in immune responses in neonates compared to adults (29). The limitations of our study were the lack of repeated neonatal RT-PCR COVID-19 sampling, placental, amniotic fluid, and membrane sampling, small sample size, and no data about maternal vitamin D deficiency and vaccination status. The present study aimed to evaluate the clinical characteristics, respiratory outcomes, and other clinical symptoms related to the infection of COVID-19 in preterm infants born to infected mothers. Long-term developmental consequences assessment for infants of COVID-19 infected mothers is recommended.
5.1. Conclusions
Based on the findings of our study, the severity of RDS, the need for surfactant replacement therapy, the duration of hospital stay, and mortality were not affected by maternal COVID-19 infection. However, the need for advanced resuscitation at birth, fever, and clinical manifestations of sepsis were associated with RDS in preterm infants born to COVID-19 infected mothers.